Induced Pluripotent Stem Cells (Ipscs) Based Liver Organoid: the Benefits and Challenges
Keywords
Abstract
Introduction
The liver, the primary metabolic organ, controls various physiological processes in the human body [1]. Approximately 70% of liver mass comprises hepatic cells (hepatocytes), which perform metabolic tasks for the liver, including chemical detoxification, plasma protein synthesis, regulation of amino acids and carbs, and the induction of inflammatory and immunological responses. The sole therapeutic option for a patient whose liver has been
damaged and has developed into a chronic condition such as end-stage liver disease (ESLD) or cirrhosis of the liver is organ transplantation [1]. Unfortunately, when it reaches its final stage, the damage to liver function is irreversible at this point, so decompensation will occur in different organs and organ systems, including lung tissue, which can result in bleeding varicose, renal damage, and ascites [2].
Increased survival rates may result from the transplantation of patients with stages 2 and 3 acute liver failure. Although more patients are being held [3], the number of those waiting for transplants continues to increase, and the supply of available transplanted organs cannot keep up with demand [4]. Expanding the availability of liver tissue replacement and creating hepatic and bioartificial liver tissue have been the main goals of the search for alternatives to whole organ transplantation [5]. Organoid culture can recreate the physiological and pathological circumstances of the tissue while preserving the DNA contained in autologous tissue [6].
Advances in regenerative medicine are opening new insights and new hope in the therapy of irreversible liver damage. Regenerative medicine for the liver focuses particularly on the development of new therapies that functionally reverse damage to existing liver tissue or create an entirely new organ. The advancement of liver regenerative medicine has benefited from the understanding of cellular and molecular principles relating to organogenesis and regeneration over the past few decades. Regenerative medicine, when compared to current operational therapy; therefore, it is less intrusive, less expensive, and a solution to donor limits, immunological rejection, and other issues. The ideal treatment for liver cirrhosis is liver regeneration therapy [7].
The latest technologies in cellular and molecular research are rapidly developing to meet the needs of clinical applications that involve the diagnosis and treatment of liver disease.
[8] Several recent studies found that the notion of co-culturing induced pluripotent stem cells with diverse nonparenchymal cell supporting cells can acquire higher differentiation and develop and boost the function of hepatocyte cells produced [9]. In this approach, some researchers employ bioengineering to construct liver organoids derived from a decellularized liver [10].
Human-induced pluripotent stem cells’ ability to differentiate into several hepatic cell lineages
For several years, scientists have worked to promote the three main lineages of the germ layers in vitro development from basic, possibly pluripotent cells. During embryological development, to recreate the necessary molecular and cellular signaling, the traditional strategy for building protocols that imitate patterns and phases was employed. Most important protocols either use one of the numerous cellular aggregation techniques or encourage the cultural differentiation as a monolayer when dealing with pluripotent cells. It is possible that over time, in what seems to be a random process, EB develops the regional differentiation. One strategy involves using various techniques, such as transplanting three-dimensional (3D) gel constructs made of Matrigel into immunodeficient animals and combining them with other cells to create an endoderm that is steered to the liver. One of these two small molecules causes enhanced adult marker expression and functional activity, including cytochrome P450s, whereas the other reduces the fetal marker expression [11].
Different development procedures, including those for hepatocytes made from human iPSCs, have been created. For this stage, an essential prerequisite is to use Activin A for the initial induction of definitive endoderm (DE). In the hepatic specification and throughout various stages of protocol development, culture media, and extra growth factors are also used as additional components. The results show that different techniques of differentiation have varying degrees of success; for instance, after 16–25 days of human iPSC hepatic
differentiation, positive adult hepatocyte cells can be identified by the presence of albumin or Alpha 1 antitrypsin [7]. Brief exposure to the factor Wnt3a, which is expressed at the human heart’s developmental stage and interacts specifically with activin A, can increase DE and hepatic output. At a later stage of embryonic development, fibroblast growth factors 2 (FGF2) work in conjunction with bone morphogenic protein-4 (BMP4) to aid in the production of DE cells. Another important finding is that in the presence of serum from cow fetuses in culture media, the effects of all the factors that stimulate the development of early mammals are inhibited. This approach will produce functionally enhanced hepatocyte-like cells. Different marker genes expressed out of DE during embryonic development include SRY, Sox17, and Foxa2 [7, 12].
The next step toward hepatocyte formation is the induction from DE of the development of liver progenitor cells or hepatoblasts. In cell culture, induction is accomplished by introducing a certain growth factor, with hepatocyte growth factor (HGF) serving as the primary one. During the liver development process, hepatocyte nuclear factor 4 alpha (HNF4α) expression will increase and mark differentiation toward the hepatocyte line during in vitro differentiation. The essential marker of the liver progenitor cells is the α-fetoprotein (AFP) serum glycoprotein, which is expressed by primordial hepatocytes [7].
The last step in the differentiation process is induced by hepatocyte maturation. This was obtained in cell culture by adding Oncostatin M (OSM), which in combination with glucocorticoids is a cytokine from the interleukin-6 family to cell culture media. Morphological observations such as hepatocytes and intracellular glycogen production are consistent with a cell-like differentiation of the hepatocytes. Strategies to change the cells of the line of descent heart of phenotype hepatoblasts or hepatocytes on a fetus into a cell with characteristics of adult primary hepatocytes [7].
The efficiency of differentiation for each type of cell phase was measured using the hepatic stage-specific marker, with the results obtained being efficiencies reliably greater than 90% (up to 98%) for the second phase of the cell endoderm and the definitive progenitor liver but more variable (between 50% and 90%) for the final phase, that is, in adult cells such as hepatic cells [7]. Variations in culture conditions, including cell culture, density before and after the induction of endoderm, and a selection of essential growth agents and substances, can significantly affect each differentiation stage’s efficacy. Examples include the size of iPSC colonies that are not differentiated at the time of differentiation initiation, iPSC colony culture, and cell culture [7, 12-14].
In 3D co-culture, the main hepatocytes and the epithelial sinusoidal liver cells assist each other. The concept has been extended to the hepatocyte-like cells derived from the iPSC, which shows that the co-cultured endothelial cells and stromal support the maturation of cells such as hepatocytes by touch cells or by paracrine factor. Specific DE hepatocytes have recently been cultured with human umbilical vein endothelial cells and mesenchymal stem cells (MSCs), which can produce 3D cell clusters in which iPSC-derived cells express genes that connect to other hepatocytes, such as AFP and albumin, suggesting that cluster formation may speed up hepatocyte maturation [15].
There has been a lot of use of hepatocyte reference controls such as cell lines or cultured hepatocytes and a population standard that can be replicated, but these lines do not represent the proper physiological level of the particular function of hepatocytes. Therefore, primary human hepatocytes cryopreserved, are freshly isolated, or are not cultured are usually considered ideal reference controls. Although all work quickly degrades after being stored in traditional culture systems, certain functional research calls for cells to be cultivated for several hours to days. These studies include those involving morphology, phenotypes, and primary human hepatocytes [16].
Based on the work ofMa et al. that has been conducted, , there are studies on the efficient differentiation of iPSC against homogeneous functional hepatocyte populations [17]. Factors of liver transcription, such as HNF3β, GATA4, C/EBPш, C/EBPβ, and BMP signaling (BMP-2 and BMP-4) have significant involvement in the differentiation of hepatocytes and the development of the liver. These factors were expressed throughout differentiation, which was a key element in improving the ability of these cells to differentiate into hepatocytes later on.
[18] A particular group of liver genes, including albumin, 1-AT, tyrosine aminotransferase, and G-6-P, which are more mature indicators of hepatocytes and have significant roles in the liver, will be expressed by human iPSC-derived hepatocytes (hiHs). Besides functional testing, hiHs exhibit glycogen accumulation, indocyanine green (ICG) absorption and excretion, and albumin secretion into the medium. These results suggest that the time needed for hiHs to produce albumin at a level comparable to that of hESC-derived hepatocytes (20–22 days) is longer (26–27 days after differentiation). It represents the initial difference in hESC–hiPSC differentiation [18, 19].
There are vast variations in liver differentiation protocols, diagnostic methods, and phenotypic or functional outcomes. Hence, comparing the effectiveness of the pluripotent stem cell pathways to produce functional hepatocytes precisely is challenging. The hiPSC line, as a general observation, presents differentiation and performance of the hepatocytes more varied than the ESC line, specifically the H9 line. This is because the available protocol differentiation has been developed to track the ESC, and the protocol is not intended to discuss potential memory epigenetics that are maintained or irregularities that may be owned by the iPSC track, ensuring that the iPSC path responds to weak heart differentiation signals [20, 21].
How can organoids research benefit end-stage liver disease?
Heaps of the current research is behind the successful advances in medical treatment using cancer cell or animal cell models, both of which have drawbacks, especially concerning medical applications. Nevertheless, the development of stem cell biology has been able to culture tissues that resemble the liver and intestines over a long period from local tissue or pluripotent stem cells [15]. Three-dimensional structures, called organoids, represent important structures and complexity of function beyond the frequent and changing traditional in vitro cell culture models [22].
Both the ideal immunosuppressive protocols and the most effective cellular combination that may engraft and multiply over an extended period must be properly identified. Numerous studies demonstrated that diverse cell types can be created from induced pluripotent stem cells (iPSCs), including hepatocytes, cholangiocytes, endothelial cells, and Kupffer cells, which are all found in the liver. The development of useful bioartificial livers has been facilitated by the growing interest in the study of 3D organoids that self-assemble or are driven by matrices [23]. The various cell types that make up the liver should be present in a realistic liver replica, and the spatial arrangement of these cells should resemble that of the tissue architecture [24].
The liver was successfully encapsulated into alginate material and it survived and functioned well when transplanted into immunocompetent mice. Better cell models and creating transplantable human tissue are prerequisites for establishing a renewable source of liver tissue [25].. The manufacture of human embryonic hESCs offers fascinating opportunities for the advancement of biology, models for diseases, and cell treatment [26]. Significant progress toward achieving this goal is hampered by the dependence on the ingredients used of animal origin (e.g., Matrigel), immortal cell lines, and their resulting forms that are difficult to control and reproduce [27].
Presently, organoid technology has developed into an art in cell culture to study human biology in the world of health and certain diseases. Generally, organoids can simply be said to be mini-structures of organs in a 3D form that can develop into a 3D matrix in which the aspects
of the structure and function are the same as the real organs. Organoids have been extensively exploited as brand-new therapeutic candidates for things such as illness modeling and medication discovery [2]. Exogenous factors promote cell self-renewal, proliferation, and differentiation via inducing several signaling pathways and support tissue-specific organogenesis, all of which contribute to the production of organoids [28]. Producing liver organoids using bioengineering can produce more physiologically realistic and biomedically useful liver organoids [29].
Disease models in the form of organoids have been developed substantially, such as acquired disorders connected to toxin products that have an organoid model and hereditary high cholesterol has been better defined; this has allowed for greater knowledge and research of already prescribed medications as well as the identification of new medications [2]. The diversity of functions performed by the human liver, the usefulness of liver organoids in the modeling of many liver illnesses, and the possibility of using liver organoids as cell-based therapies in regenerative medicine make them of particular interest. Organoid models also open up new avenues for personalized medicine and drug development because they may be created from patient tissues [30]. An important turning point in the modeling of liver illness is the use of liver organoids, which transcend the limitations of bidimensional culture and the high expense of in vivo models [31]. Access to multicellular organotypic surrogate models for illness, toxicity, and drug development would be considerably enhanced with interactions and architecture that are similar to those in vivo. Organoids and microfluidic chip technologies would be combined in this, potentially leading to new therapeutic strategies [32].
Human-induced pluripotent stem cell-based 3D liver organoid production
Organoids are a 3D culture system that uses adult stem cells and their offspring to develop and represent the physiological state of the cell under in vivo conditions [3]. The formation of liver organoids from IPSc was first introduced by Takebe et al. using co-culture. In his model, human MSCs and human umbilical vein endothelial cells were co-cultured with liver progenitor cells that were produced through the progressive differentiation of IPSC in two-dimensional (2D) cell cultures (HUVECs). Then, on a microscope, IPSC-liver buds (LB), which spontaneously formed on culture in Matrigel, were visible. Additionally, the presence of a connection to the host vessel at the moment of transplant makes the human blood vessel structure in the IPSC-LB functional. Particularly, albumin secretion into blood arteries by liver cells implanted in LB started from days 10 to 45 after transplantation in mice. The bud organs’ structure, which shows regenerating powers and prevents death from liver failure, is more significant [33].
The primary objective anticipated by the researchers is to produce functional liver organoids by combining current techniques with the advancement of bioengineering technology. The development of “organs-on-a-chip,” bioengineered miniature organs relies on exact manipulation of the environment for hepatocyte function. Frequently, mature hepatocytes derived directly from humans are used in this approach. The chip’s ability to mechanically manage the spatiotemporal interactions of the various cell types is made possible by the incorporation of many cell types. Microfluidics and soft lithography technology have created the culture environment, which frequently results in a static culture environment. The precise regulation of input (drugs, nutrients, and oxygen) and output (metabolites, biosensing, and electrical potential) made possible by combining technology and bioengineering will have many benefits and improve liver function. A mix of extracellular signals that are inductive and repressive at different concentrations determines the fate of the human liver [7, 34, 35].
The generated hepatocyte-like cells have unique secretory properties (albumin and urea) and drug-metabolizing capabilities. They can also absorb ICG and store lipids and glycogen (CYP3A4 activity); the system’s biliary structure demonstrates gamma-glutamyltransferase activity, the ability to release rhodamine, and the capacity to store bile acids [36].
Mesenchymal stromal cell and iPS-derived endothelial cell liver organoids exhibit considerable albumin expression and production, elevated CYP1A1, CYP1A2, and TDO2 expression, and decreased TGF-beta and Wnt signaling activity. Large changes in protein expression caused by a particular combination of nonparenchymal cells were discovered by proteomic analysis to be connected to integrin profiles and TGF-beta/Wnt signaling activity [37, 38].
Hepatocytes, liver sinusoidal endothelial cells, and MSCs are a mixture of human cells that, when cultivated in vitro on a thick layer of Matrigel TM, self-regulate to create structures that mimic liver organoids in 24 h. After being cultivated for up to 10 days in a bioreactor, these liver organoids display different functional characteristics such as hepatic parenchymal cells, the activity of cytochromes P450, CYP3A4, CYP2B6, and CYP2C9, and mRNA expression on multiple marker genes and other enzymes [38].
Three-dimensional culture systems and liver organoids
An interesting approach to long-term 3D cell culture is a technique introduced in the late 1990s. A technique created by Broutier et al. produces organoid livers using EpCam and ductal cells from human livers that are either healthy or injured. It can develop into a 3D structure with progenitor biliary cells that enable them to differentiate into liver lineages when cultured in Matrigel or using a low attachment plate with EGF, HGF, FGF, and RSPO1. The benefit of this technique over IPSC-derived organoids is that these organoids may be cryopreserved and cultivated for an extended period of time (up to more than a year) while maintaining genetic stability. This facilitates the cell banking procedure [39].
Invagination of the 3D culture of pluripotent stem cells of human origin, along with a continuous and dynamic pattern of structural morphogenesis of the liver, biliary, and pancreatic, was described by Koike et al. In the absence of external stimuli, retinoic acid- dependent formation of hepatobiliary–pancreatic organ domains designated at the foregut– midgut boundary organoids is enabled by the interaction between anterior and posterior gut spheroids produced from pluripotent stem cells of human origin [40].
Akbari et al. developed and characterized hepatic organoids (eHEPO) that can be produced in 2 weeks and multiplied over more than 16 months without losing the capacity to become mature hepatocytes. Table 1-4 show the various culture methods with the combination of cells used and the medium and supplementation used to produce a functional organoid liver in the last 3 years. From this tables, we can see that the generation of the organoid liver can be produced with various technologies and requires various supplements [41].
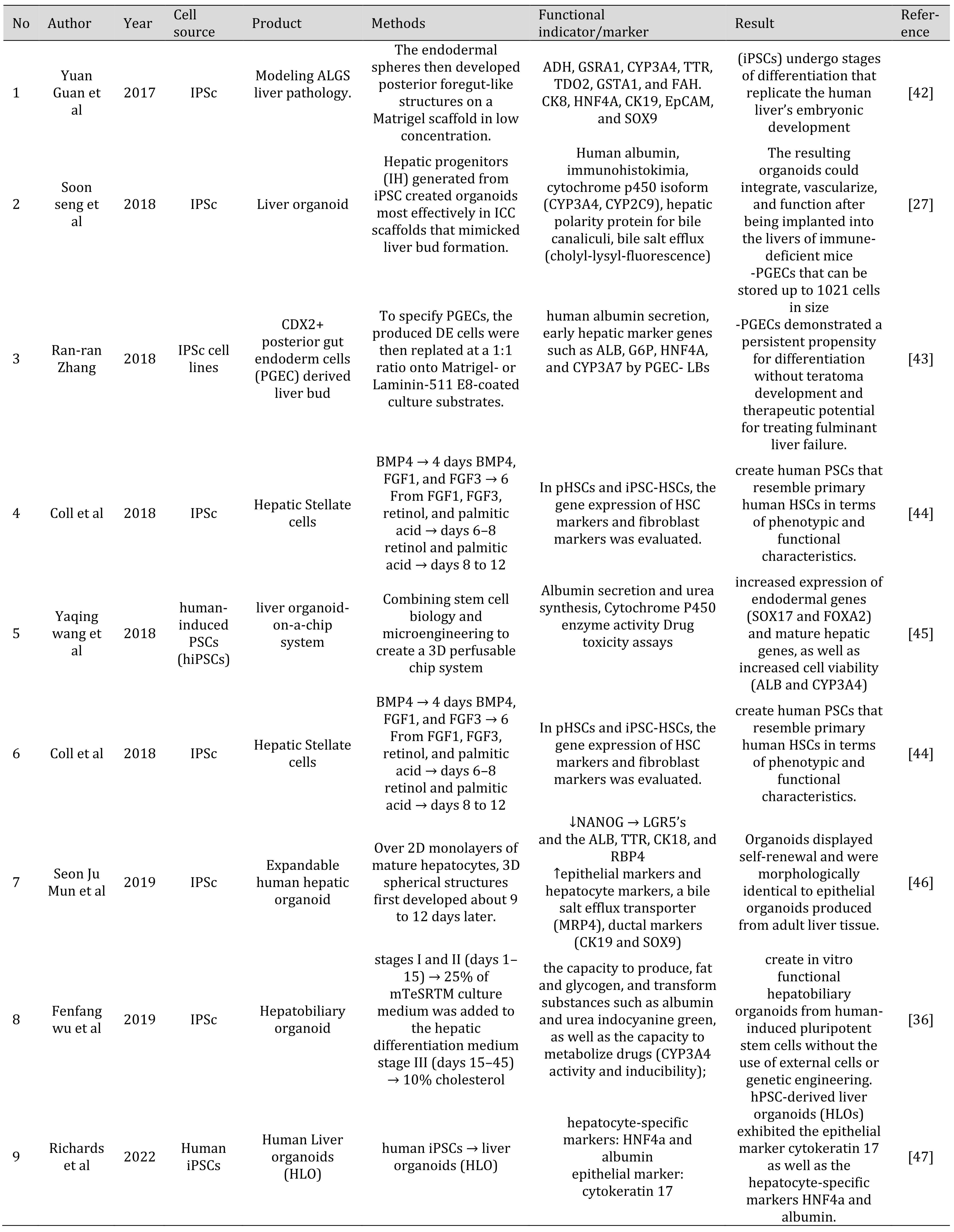
Table 1: Various methods to generate liver organoid from IPSC

Table 2: Various methods to generate liver organoid from IPSC and coculture with other cells
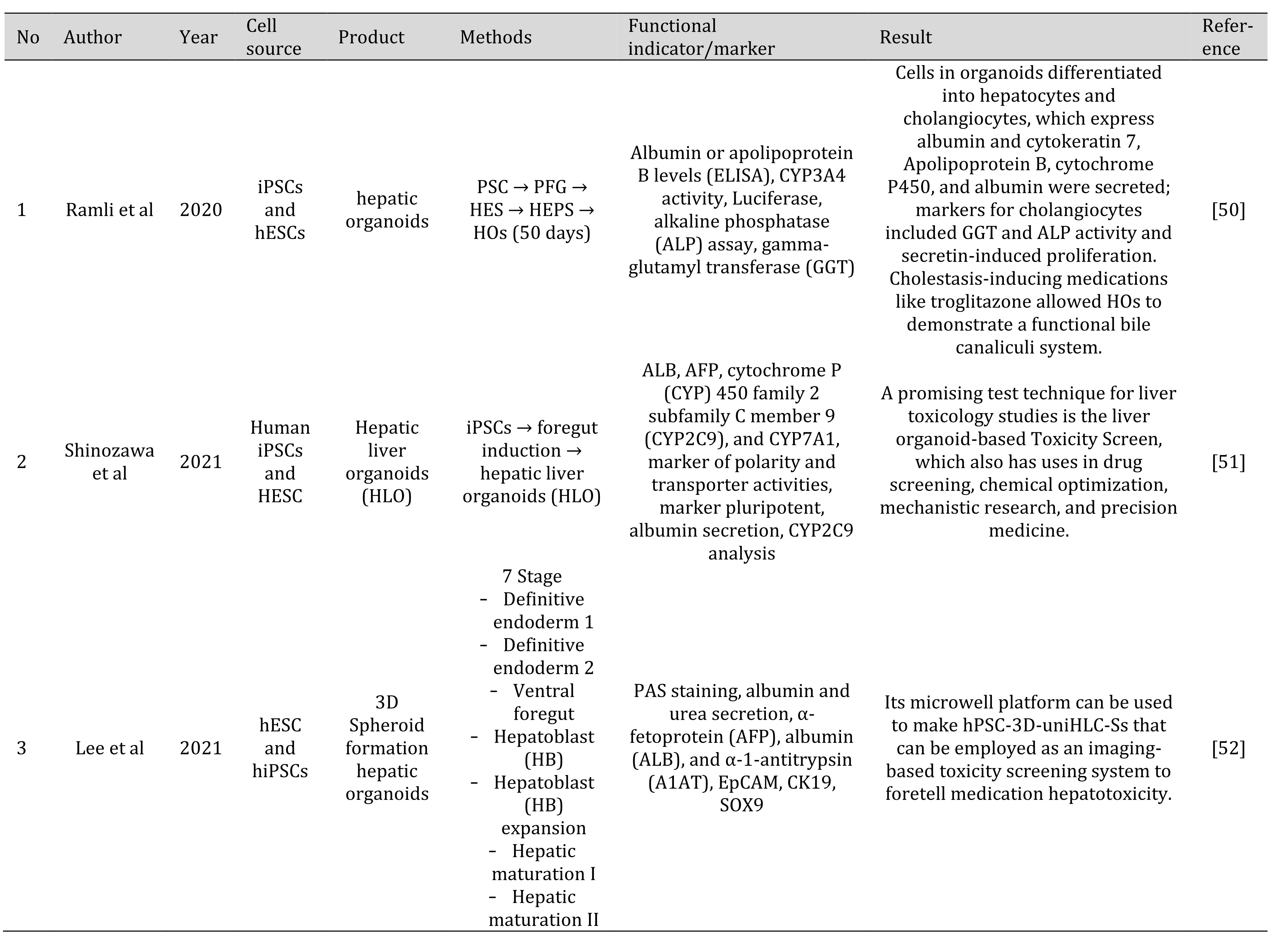
Table 3: Various methods to generate liver organoid from IPSC and human Embryonic Stem Cells (hESCs)

Table 4: Various methods to generate liver organoid from primary cells
Comparison between 2D and 3D organoid
The genetic landscape and histoarchitecture could not be represented by cells cultivated in two dimensions. Researchers have been successful in creating culture conditions for the organoid system to produce a better model that can imitate the histoarchitecture and genetic landscape of liver cancer. Organoids are characterized by self-structuring 3D structures that respond to the original architecture of the organ and/or tumor in the in vivo state and can be constructed from multiple sources. Organoids can currently be created from adult stem cells that are unique to a particular organ, as well as from embryonic or induced pluripotent stem cells, tumor cells, or adult stem cells (referred to as tumoroids) [3].
The use of 3D systems has numerous benefits, including the ability to better sustain cell-to-cell contact, which allows cells to communicate directly with one another and to aggregate on their own to form the organization of the cells in the organ. Most of these techniques used the Matrigel matrix. It is now widely accepted that cells cultured using the 3D method are more similar in architecture to function in living tissue than cells cultured using the 2D technique. One of the causes is the development of cell-to-cell contacts and connections between cells and the extracellular matrix in 3D cultures, whereas these interactions are only possible in 2D cultures in the horizontal plane. Cells in tissues are frequently exposed to stratified concentrations of effector signal molecules, nutrients, and waste products, similar to a 3D culture system where the cells located in the middle of the aggregates or organoids have limited access to the components present in the culture medium. Contrarily, since all cells in 2D monolayer cultures were in direct contact with the culture medium, they were all exposed to the same concentration of the components present in the medium. To modify cell survival, migration, morphogenesis, differentiation, proliferation, and differentiation, it is discovered that the construction of a microenvironment using 3D approaches is more advantageous physiologically, biochemically, and biomechanically [22, 29].
Monolayer culture, also known as 2D flat culture, has been commonly used in the early stages of drug screening. In addition to modifying the distribution of the different cell types in a regulated way, micropatterned surfaces also offer biochemical signals for both parenchymal and nonparenchymal slopes. Table 5 shows the various comparison between 2D and 3D methodsin liver organoids for the last 3 years [58].
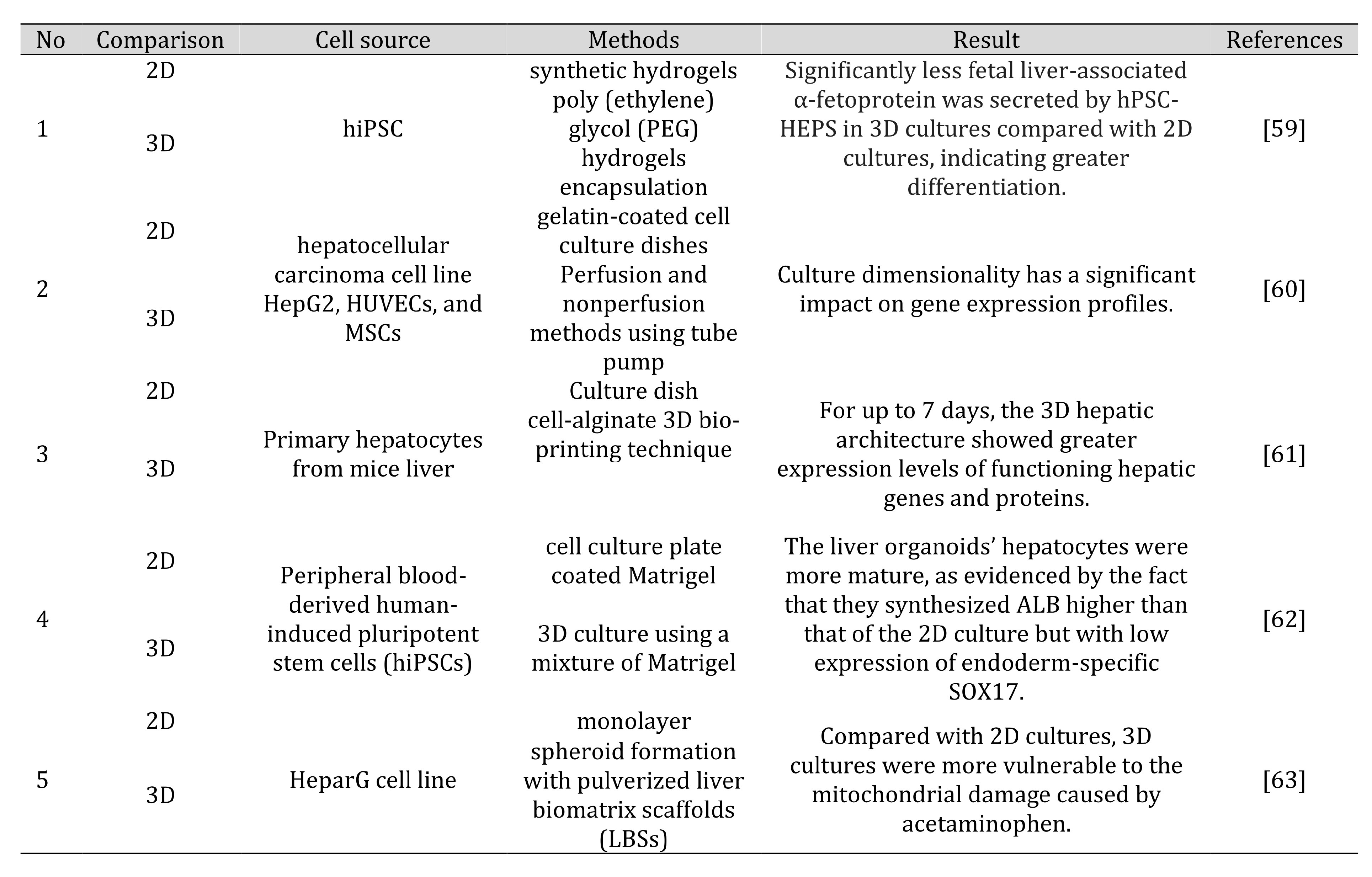
Table 5: Various comparisons of 2D and 3D methods in liver organoids
Translational research challenges the clinical application of liver
One of the more alluring methods for treating people with liver failure is hepatocyte transplantation. This is because liver failure patients’ human-induced pluripotent stem cell- derived hepatocyte-like cells (iPS-HLCs), which are anticipated to be used for hepatocyte transplantation, can be created on a massive scale. iPSCs are an excellent source for an in vitro model of liver disease, as per various protocols to produce hepatic cells from them. They also represent a significant advancement in the field of liver disease since samples taken from patients during surgery or liver transplantation were once a potential source of primary cells [22, 64].
An extracellular matrix that may induce cell aggregation into 3D structures is necessary for creating organoid livers. Most organoid cultures’ compositions, including those of the liver, are challenging to explain, and several variations are the result of cultural adaptation. This is a drawback to its potential use in several therapeutic applications and regenerative medicine. Further effort is required as synthetic platforms develop bioengineering that can aggregate liver cells in a 3D structure without losing their specifications. Another limitation is that the organoid liver is not completely in its entirety and the various types of cells present in the liver and their degree of regularity. The direct clinical application of organoid technology still faces various challenges. The transformation toward malignancy in organoids originating from stem cells is of particular after transplanting and thorough animal model observation is a way of dealing with this problem [22].
Finding the best way to produce organoids is another difficult task. Although the current technique for organoid cultivation using an extracellular matrix (Matrigel) and bioengineered factor creation is crucial, it involves components of animal origin (bovine serum). This substance may contaminate organoid products and produce adverse effects on human hosts. The use of bioproducts without chemical explanation has a negative impact, so in the future, bioengineering techniques are necessary to produce organoids using methods that can be explained chemically [65].
Determining the proper cell type and creating vascular tissue that will enable cell aggregation provide significant challenges for organ bioengineering. The determination of cell types, ideal cell volume, and seeding techniques are the major challenges in producing vital liver functions. To better understand how liver cells may organize themselves to produce the liver, one of the body’s most complicated organs, these fascinating difficulties call for a multidisciplinary approach combining biologists, doctors, and bioengineers [7].
In recent years, organoid 3D cultures of various tumor subtypes and organs have been successfully developed, paving the way for tumor research. Organoid 3D culture is used to explain tumor diversity, to model cancer in the lab, and to determine response to therapy. A promising strategy to combat drug resistance and improve the effectiveness of tailored anticancer therapies is creating a biobank and creating a next-generation matrix [3].
Although the current progress is very significant, there are still unmet clinical needs. This encourages the development of alternative treatments for diseases and impaired liver function. A critical issue that has yet to be resolved is how to accelerate the translation of cell therapy into clinical practice, which is a prerequisite for liver transplant recipients to increase engraftment and proliferation of donor cells, and the development of noninvasive and tracking methods to monitor accurate cell survival and processes [66].
Regenerative medicine for the human liver has seen numerous significant advancements. This includes the development of a replicable cell source and the prospect of overcoming low donor organ and hepatocyte stability, which is an immune system challenge. Although there have been many developments, liver cells derived from stem cells require further development before they can be used clinically [67]. An interesting future is offered in this research because efforts now focus on ways to enhance cell activity, engraftment, and
stability. Deeper research has been conducted on alternative medicines, xeno-organ, scaffold- based, and specific cell transplantation. Hepatocytes and MSCs have been used in cell transplantation alone in clinical settings. Xeno-organ and cell transplantation are combined in a thorough procedure known as scaffold-based transplantation. Clinical applications of scaffold-based transplantation will be the subject of future study [68].
For regenerative medicine to be successful, hiPSC-based LO must be safe in vivo. Consequently, reliable genome editing techniques, effective in vivo delivery methods, and the usage of xeno-free materials are all required. Genetic and epigenetic stability must be examined when the IPSC-based liver organoid is being created and grown. The current difficulties in disease modeling and medication development are to increase complexity, decrease heterogeneity, and improve maturity [69]. Another crucial area for future research is the well- known risk of teratoma development because of undifferentiated cell contamination following cell transplantation. Procedures that may remove nondifferentiated cells from a batch of samples created using differentiation processes must be set up before being used in a clinical context [70].
Conclusion
This review summarized alternative protocols to differentiate hepatocytes from iPSC and to generate LO based on iPSC from varying perspectives. For the growth of human IPSC cells to LO, numerous procedures have been created. Future studies are still anticipated to focus on the clinical use of LO in ESLD patients.
Acknowledgements
The authors would like to thank Research Grant Publikasi Terindeks Internasional (PUTI) Universitas Indonesia 2022 (Batch 2).
Disclosure Statement
No conflicts of interest exist, according to the authors.
References
| 1 | Dooley JS, Lok AS, Garcia-Tsao G, Pinzani M: Sherlock's diseases of the liver and biliary system. John Wiley & Sons, 2018.
https://doi.org/10.1002/9781119237662 |
| 2 | Messina A, Luce E, Hussein M, Dubart-Kupperschmitt A: Pluripotent-Stem-Cell- Derived Hepatic Cells: Hepatocytes and Organoids for Liver Therapy and Regeneration. Cells 2020;9
https://doi.org/10.3390/cells9020420 |
| 3 | Tharehalli U, Svinarenko M, Lechel A: Remodelling and Improvements in Organoid Technology to Study Liver Carcinogenesis in a Dish. Stem Cells Int 2019;2019:3831213.
https://doi.org/10.1155/2019/3831213 |
| 4 | Kuse Y, Taniguchi H: Present and Future Perspectives of Using Human-Induced Pluripotent Stem Cells and Organoid Against Liver Failure. Cell Transplant 2019;28:160s- 165s.
https://doi.org/10.1177/0963689719888459 |
| 5 | Schwartz RE, Bram Y, Frankel A: Pluripotent stem cell-derived hepatocyte-like cells: a tool to study infectious disease. Current pathobiology reports 2016;4:147-156.
https://doi.org/10.1007/s40139-016-0113-7 |
| 6 | Wu L-J, Chen Z-Y, Wang Y, Zhao J-G, Xie X-Z, Chen G: Organoids of liver diseases: From bench to bedside. World journal of gastroenterology 2019;25:1913.
https://doi.org/10.3748/wjg.v25.i16.1913 |
| 7 | Ang LT, Tan AKY, Autio MI, Goh SH, Choo SH, Lee KL, Tan J, Pan B, Lee JJH, Lum JJ: A roadmap for human liver differentiation from pluripotent stem cells. Cell reports 2018;22:2190-2205.
https://doi.org/10.1016/j.celrep.2018.01.087 |
| 8 | Torres S, Abdullah Z, Brol MJ, Hellerbrand C, Fernandez M, Fiorotto R, Klein S, Königshofer P, Liedtke C, Lotersztajn S: Recent Advances in Practical Methods for Liver Cell Biology: A Short Overview. International journal of molecular sciences 2020;21:2027.
https://doi.org/10.3390/ijms21062027 |
| 9 | Pettinato G, Lehoux S, Ramanathan R, Salem MM, He LX, Muse O, Flaumenhaft R, Thompson MT, Rouse EA, Cummings RD, Wen X, Fisher RA: Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Sci Rep 2019;9:8920.
https://doi.org/10.1038/s41598-019-45514-3 |
| 10 | Tayyeb A, Azam F, Nisar R, Nawaz R, Qaisar U, Ali G: Regenerative medicine in liver cirrhosis: promises and pitfalls. Liver Cirrhosis-Update and Current Challenges 2017:236- 256.
https://doi.org/10.5772/intechopen.68729 |
| 11 | Gallicano GI, Mishra L: Hepatocytes from induced pluripotent stem cells: a giant leap forward for hepatology. Hepatology (Baltimore, Md) 2010;51
https://doi.org/10.1002/hep.23474 |
| 12 | Palakkan AA, Nanda J, Ross JA: Pluripotent stem cells to hepatocytes, the journey so far. Biomed Rep 2017;6:367-373.
https://doi.org/10.3892/br.2017.867 |
| 13 | Schwartz R, Fleming H, Khetani S, Bhatia S: Pluripotent stem cell-derived hepatocyte- like cells. Biotechnology advances 2014;32:504-513.
https://doi.org/10.1016/j.biotechadv.2014.01.003 |
| 14 | Sauer V, Tchaikovskaya T, Wang X, Li Y, Zhang W, Tar K, Polgar Z, Ding J, Guha C, Fox IJ, Roy-Chowdhury N, Roy-Chowdhury J: Human Urinary Epithelial Cells as a Source of Engraftable Hepatocyte-Like Cells Using Stem Cell Technology. Cell Transplant 2016;25:2221-2243.
https://doi.org/10.3727/096368916X692014 |
| 15 | Blackford SJI, Ng SS, Segal JM, King AJF, Austin AL, Kent D, Moore J, Sheldon M, Ilic D, Dhawan A, Mitry RR, Rashid ST: Validation of Current Good Manufacturing Practice Compliant Human Pluripotent Stem Cell-Derived Hepatocytes for Cell-Based Therapy. Stem Cells Transl Med 2019;8:124-137.
https://doi.org/10.1002/sctm.18-0084 |
| 16 | Du C, Narayanan K, Leong MF, Wan AC: Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials 2014;35:6006-6014.
https://doi.org/10.1016/j.biomaterials.2014.04.011 |
| 17 | Ma X, Duan Y, Tschudy-Seney B, Roll G, Behbahan IS, Ahuja TP, Tolstikov V, Wang C, McGee J, Khoobyari S, Nolta JA, Willenbring H, Zern MA: Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells. Stem Cells Transl Med 2013;2:409-419.
https://doi.org/10.5966/sctm.2012-0160 |
| 18 | Ma X, Duan Y, Tschudy-Seney B, Roll G, Behbahan IS, Ahuja TP, Tolstikov V, Wang C, McGee J, Khoobyari S: Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells. Stem cells translational medicine 2013;2:409-419.
https://doi.org/10.5966/sctm.2012-0160 |
| 19 | Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA: Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 2010;51:297-305.
https://doi.org/10.1002/hep.23354 |
| 20 | Yu Y, Liu H, Ikeda Y, Amiot BP, Rinaldo P, Duncan SA, Nyberg SL: Hepatocyte-like cells differentiated from human induced pluripotent stem cells: relevance to cellular therapies. Stem cell research 2012;9:196-207.
https://doi.org/10.1016/j.scr.2012.06.004 |
| 21 | Hay DC, Zhao D, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, Black JR, Elcombe C, Ross JA, Wolf R: Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem cells 2008;26:894-902.
https://doi.org/10.1634/stemcells.2007-0718 |
| 22 | Fiorotto R, Amenduni M, Mariotti V, Fabris L, Spirli C, Strazzabosco M: Liver diseases in the dish: iPSC and organoids as a new approach to modeling liver diseases. Biochim Biophys Acta Mol Basis Dis 2019;1865:920-928.
https://doi.org/10.1016/j.bbadis.2018.08.038 |
| 23 | Olgasi C, Cucci A, Follenzi A: iPSC-derived liver organoids: a journey from drug screening, to disease modeling, arriving to regenerative medicine. International journal of molecular sciences 2020;21:6215.
https://doi.org/10.3390/ijms21176215 |
| 24 | Lam DTUH, Dan YY, Chan Y-S, Ng H-H: Emerging liver organoid platforms and technologies. Cell Regeneration 2021;10:1-19.
https://doi.org/10.1186/s13619-021-00089-1 |
| 25 | Günther C, Brevini T, Sampaziotis F, Neurath MF: What gastroenterologists and hepatologists should know about organoids in 2019. Dig Liver Dis 2019;51:753-760.
https://doi.org/10.1016/j.dld.2019.02.020 |
| 26 | Lucendo-Villarin B, Rashidi H, Alhaque S, Fischer L, Meseguer-Ripolles J, Wang Y, O'Farrelly C, Themis M, Hay DC: Serum Free Production of Three-dimensional Human Hepatospheres from Pluripotent Stem Cells. JoVE (Journal of Visualized Experiments) 2019:e59965.
https://doi.org/10.3791/59965-v |
| 27 | Ng SS, Saeb-Parsy K, Blackford SJ, Segal JM, Serra MP, Horcas-Lopez M, Mastoridis S, Jassem W, Frank CW, Cho NJ: Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials 2018;182:299-311.
https://doi.org/10.1016/j.biomaterials.2018.07.043 |
| 28 | Akbari S, Arslan N, Senturk S, Erdal E: Next-Generation Liver Medicine Using Organoid Models. Front Cell Dev Biol 2019;7:345.
https://doi.org/10.3389/fcell.2019.00345 |
| 29 | Prior N, Inacio P, Huch M: Liver organoids: from basic research to therapeutic applications. Gut 2019;68:2228-2237.
https://doi.org/10.1136/gutjnl-2019-319256 |
| 30 | Nuciforo S, Heim MH: Organoids to model liver disease. JHEP Reports 2021;3:100198.
https://doi.org/10.1016/j.jhepr.2020.100198 |
| 31 | Caiazza C, Parisi S, Caiazzo M: Liver organoids: Updates on disease modeling and biomedical applications. Biology 2021;10:835.
https://doi.org/10.3390/biology10090835 |
| 32 | Harrison SP, Baumgarten SF, Verma R, Lunov O, Dejneka A, Sullivan GJ: Liver organoids: Recent developments, limitations and potential. Frontiers in medicine 2021;8:574047.
https://doi.org/10.3389/fmed.2021.574047 |
| 33 | Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang R-R, Ueno Y, Zheng Y-W, Koike N: Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013;499:481-484.
https://doi.org/10.1038/nature12271 |
| 34 | Takebe T, Zhang B, Radisic M: Synergistic engineering: organoids meet organs-on-a- chip. Cell Stem Cell 2017;21:297-300.
https://doi.org/10.1016/j.stem.2017.08.016 |
| 35 | Asai A, Aihara E, Watson C, Mourya R, Mizuochi T, Shivakumar P, Phelan K, Mayhew C, Helmrath M, Takebe T, Wells J, Bezerra JA: Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development 2017;144:1056- 1064.
https://doi.org/10.1242/dev.142794 |
| 36 | Wu F, Wu D, Ren Y, Huang Y, Feng B, Zhao N, Zhang T, Chen X, Chen S, Xu A: Generation of hepatobiliary organoids from human induced pluripotent stem cells. J Hepatol 2019;70:1145-1158.
https://doi.org/10.1016/j.jhep.2018.12.028 |
| 37 | Goulart E, de Caires-Junior LC, Telles-Silva KA, Araujo BHS, Kobayashi GS, Musso CM, Assoni AF, Oliveira D, Caldini E, Gerstenhaber JA, Raia S, Lelkes PI, Zatz M: Adult and iPS- derived non-parenchymal cells regulate liver organoid development through differential modulation of Wnt and TGF-β. Stem Cell Res Ther 2019;10:258.
https://doi.org/10.1186/s13287-019-1367-x |
| 38 | Ramachandran SD, Schirmer K, Münst B, Heinz S, Ghafoory S, Wölfl S, Simon-Keller K, Marx A, Øie CI, Ebert MP, Walles H, Braspenning J, Breitkopf-Heinlein K: In vitro Generation of Functional Liver Organoid-Like Structures Using Adult Human Cells. PLoS One 2015;10:e0139345.
https://doi.org/10.1371/journal.pone.0139345 |
| 39 | Mattei G, Magliaro C, Giusti S, Ramachandran SD, Heinz S, Braspenning J, Ahluwalia A: On the adhesion-cohesion balance and oxygen consumption characteristics of liver organoids. PLoS One 2017;12:e0173206.
https://doi.org/10.1371/journal.pone.0173206 |
| 40 | Koike H, Iwasawa K, Ouchi R, Maezawa M, Giesbrecht K, Saiki N, Ferguson A, Kimura M, Thompson WL, Wells JM, Zorn AM, Takebe T: Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature 2019;574:112-116.
https://doi.org/10.1038/s41586-019-1598-0 |
| 41 | Akbari S, Sevinç GG, Ersoy N, Basak O, Kaplan K, Sevinç K, Ozel E, Sengun B, Enustun E, Ozcimen B, Bagriyanik A, Arslan N, Önder TT, Erdal E: Robust, Long-Term Culture of Endoderm-Derived Hepatic Organoids for Disease Modeling. Stem Cell Reports 2019;13:627- 641.
https://doi.org/10.1016/j.stemcr.2019.08.007 |
| 42 | Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, Funayama S, Nakanishi N, Hisai T, Kobayashi T, Kasai T, Kitada R, Mori A, Ayabe H, Ejiri Y, Amimoto N, Yamazaki Y, Ogawa S, Ishikawa M, Kiyota Y, Sato Y, Nozawa K, Okamoto S, Ueno Y, Taniguchi H: Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep 2017;21:2661-2670.
https://doi.org/10.1016/j.celrep.2017.11.005 |
| 43 | Schepers A, Li C, Chhabra A, Seney BT, Bhatia S: Engineering a perfusable 3D human liver platform from iPS cells. Lab Chip 2016;16:2644-2653.
https://doi.org/10.1039/C6LC00598E |
| 44 | Guan Y, Xu D, Garfin PM, Ehmer U, Hurwitz M, Enns G, Michie S, Wu M, Zheng M, Nishimura T, Sage J, Peltz G: Human hepatic organoids for the analysis of human genetic diseases. JCI Insight 2017;2
https://doi.org/10.1172/jci.insight.94954 |
| 45 | Zhang R-R, Koido M, Tadokoro T, Ouchi R, Matsuno T, Ueno Y, Sekine K, Takebe T, Taniguchi H: Human iPSC-derived posterior gut progenitors are expandable and capable of forming gut and liver organoids. Stem cell reports 2018;10:780-793.
https://doi.org/10.1016/j.stemcr.2018.01.006 |
| 46 | Wang Y, Wang H, Deng P, Chen W, Guo Y, Tao T, Qin J: In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip 2018;18:3606-3616.
https://doi.org/10.1039/C8LC00869H |
| 47 | Coll M, Perea L, Boon R, Leite SB, Vallverdú J, Mannaerts I, Smout A, El Taghdouini A, Blaya D, Rodrigo-Torres D, Graupera I, Aguilar-Bravo B, Chesne C, Najimi M, Sokal E, Lozano JJ, van Grunsven LA, Verfaillie CM, Sancho-Bru P: Generation of Hepatic Stellate Cells from Human Pluripotent Stem Cells Enables In vitro Modeling of Liver Fibrosis. Cell Stem Cell 2018;23:101-113.e107.
https://doi.org/10.1016/j.stem.2018.05.027 |
| 48 | Mun SJ, Ryu JS, Lee MO, Son YS, Oh SJ, Cho HS, Son MY, Kim DS, Kim SJ, Yoo HJ, Lee HJ, Kim J, Jung CR, Chung KS, Son MJ: Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol 2019;71:970-985.
https://doi.org/10.1016/j.jhep.2019.06.030 |
| 49 | Ouchi R, Togo S, Kimura M, Shinozawa T, Koido M, Koike H, Thompson W, Karns RA, Mayhew CN, McGrath PS, McCauley HA, Zhang RR, Lewis K, Hakozaki S, Ferguson A, Saiki N, Yoneyama Y, Takeuchi I, Mabuchi Y, Akazawa C, Yoshikawa HY, Wells JM, Takebe T: Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab 2019;30:374-384.e376.
https://doi.org/10.1016/j.cmet.2019.05.007 |
| 50 | Ramli MNB, Lim YS, Koe CT, Demircioglu D, Tng W, Gonzales KAU, Tan CP, Szczerbinska I, Liang H, Soe EL: Human pluripotent stem cell-derived organoids as models of liver disease. Gastroenterology 2020;159:1471-1486. e1412.
https://doi.org/10.1053/j.gastro.2020.06.010 |
| 51 | Gómez-Mariano G, Matamala N, Martínez S, Justo I, Marcacuzco A, Jimenez C, Monzón S, Cuesta I, Garfia C, Martínez MT: Liver organoids reproduce alpha-1 antitrypsin deficiency-related liver disease. Hepatology international 2020;14:127-137.
https://doi.org/10.1007/s12072-019-10007-y |
| 52 | Guo J, Duan L, He X, Li S, Wu Y, Xiang G, Bao F, Yang L, Shi H, Gao M: A combined model of human iPSC-derived liver organoids and hepatocytes reveals ferroptosis in DGUOK mutant mtDNA depletion syndrome. Advanced Science 2021;8:2004680.
https://doi.org/10.1002/advs.202004680 |
| 53 | Shinozawa T, Kimura M, Cai Y, Saiki N, Yoneyama Y, Ouchi R, Koike H, Maezawa M, Zhang R-R, Dunn A: High-fidelity drug-induced liver injury screen using human pluripotent stem cell-derived organoids. Gastroenterology 2021;160:831-846. e810.
https://doi.org/10.1053/j.gastro.2020.10.002 |
| 54 | Lee G, Kim H, Park JY, Kim G, Han J, Chung S, Yang JH, Jeon JS, Woo D-H, Han C: Generation of uniform liver spheroids from human pluripotent stem cells for imaging-based drug toxicity analysis. Biomaterials 2021;269:120529.
https://doi.org/10.1016/j.biomaterials.2020.120529 |
| 55 | Hou C, Hu Y, Jiang H, Xu Z, Sha W, Liu J, Ren J, Yao M: Establishment of a 3D hyperuricemia model based on cultured human liver organoids. Free Radical Biology and Medicine 2022;178:7-17.
https://doi.org/10.1016/j.freeradbiomed.2021.11.023 |
| 56 | Richards A, Friesen M, Khalil A, Barrasa MI, Gehrke L, Jaenisch R: SARS-CoV-2 infection of human pluripotent stem cell-derived liver organoids reveals potential mechanisms of liver pathology. Iscience 2022:105146.
https://doi.org/10.1016/j.isci.2022.105146 |
| 57 | Tomofuji K, Fukumitsu K, Kondo J, Horie H, Makino K, Wakama S, Ito T, Oshima Y, Ogiso S, Ishii T: Liver ductal organoids reconstruct intrahepatic biliary trees in decellularized liver grafts. Biomaterials 2022:121614.
https://doi.org/10.1016/j.biomaterials.2022.121614 |
| 58 | Deng J, Wei W, Chen Z, Lin B, Zhao W, Luo Y, Zhang X: Engineered liver-on-a-chip platform to mimic liver functions and its biomedical applications: A review. Micromachines 2019;10:676.
https://doi.org/10.3390/mi10100676 |
| 59 | Blackford SJ, Tracy T, Norman MD, Syanda AM, Manolakakis M, Lachowski D, Yan Z, Guo Y, Garitta E, Riccio F: RGD density along with substrate stiffness regulate hPSC hepatocyte functionality through YAP signalling. Biomaterials 2023;293:121982.
https://doi.org/10.1016/j.biomaterials.2022.121982 |
| 60 | Mori N, Kida YS: Expression of genes involved in drug metabolism differs between perfusable 3D liver tissue and conventional 2D-cultured hepatocellular carcinoma cells. FEBS Open bio 2020;10:1985-2002.
https://doi.org/10.1002/2211-5463.12948 |
| 61 | Kim Y, Kang K, Yoon S, Kim JS, Park SA, Kim WD, Lee SB, Ryu K-Y, Jeong J, Choi D: Prolongation of liver-specific function for primary hepatocytes maintenance in 3D printed architectures. Organogenesis 2018;14:1-12.
https://doi.org/10.1080/15476278.2018.1423931 |
| 62 | Kulkeaw K, Tubsuwan A, Tongkrajang N, Whangviboonkij N: Generation of human liver organoids from pluripotent stem cell-derived hepatic endoderms. PeerJ 2020;8:e9968.
https://doi.org/10.7717/peerj.9968 |
| 63 | Zhang C, Zhang Q, Li J, Yu L, Li F, Li W, Li Y, Peng H, Zhao J, Carmichael PL: Integration of in vitro data from three dimensionally cultured HepaRG cells and physiologically based pharmacokinetic modeling for assessment of acetaminophen hepatotoxicity. Regulatory Toxicology and Pharmacology 2020;114:104661.
https://doi.org/10.1016/j.yrtph.2020.104661 |
| 64 | Nagamoto Y, Takayama K, Ohashi K, Okamoto R, Sakurai F, Tachibana M, Kawabata K, Mizuguchi H: Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol 2016;64:1068-1075.
https://doi.org/10.1016/j.jhep.2016.01.004 |
| 65 | Sakabe K, Takebe T, Asai A: Organoid Medicine in Hepatology. Clinical Liver Disease 2020;15:3.
https://doi.org/10.1002/cld.855 |
| 66 | Tolosa L, Pareja E, Gómez-Lechón MJ: Clinical Application of Pluripotent Stem Cells: An Alternative Cell-Based Therapy for Treating Liver Diseases? Transplantation 2016;100:2548-2557.
https://doi.org/10.1097/TP.0000000000001426 |
| 67 | Alwahsh SM, Rashidi H, Hay DC: Liver cell therapy: is this the end of the beginning? Cellular and Molecular Life Sciences 2018;75:1307-1324.
https://doi.org/10.1007/s00018-017-2713-8 |
| 68 | Furuta T, Furuya K, Zheng YW, Oda T: Novel alternative transplantation therapy for orthotopic liver transplantation in liver failure: A systematic review. World J Transplant 2020;10:64-78.
https://doi.org/10.5500/wjt.v10.i3.64 |
| 69 | Chang M, Bogacheva MS, Lou Y-R: Challenges for the applications of human pluripotent stem cell-derived liver organoids. Frontiers in Cell and Developmental Biology 2021;9:748576.
https://doi.org/10.3389/fcell.2021.748576 |
| 70 | Messina A, Luce E, Hussein M, Dubart-Kupperschmitt A: Pluripotent-stem-cell- derived hepatic cells: hepatocytes and organoids for liver therapy and regeneration. Cells 2020;9:420.
https://doi.org/10.3390/cells9020420 |