Optimisation of Blood Donor Nutrition: Blood Donor Health Improvement Studies
bRegional Centre for Blood Donation and Blood Treatment named after John Paul II in Słupsk, Słupsk, Poland
Keywords
Abstract
This review provides an analysis of the current literature on the health and nutrition of blood donors, examining key aspects that affect the quality of donated blood and the well-being of donors. The review discusses effective iron absorption facilitated by key nutrients and presents evidence on the importance of a balanced diet rich in essential nutrients, such as vitamin B12 and folic acid. The review examines the differences in iron levels between men and women and highlights the role of sex hormones in regulating iron metabolism. In addition, the review discusses the link between psycho-emotional well-being and diet, showing that proper dietary habits can improve mental health, reduce stress, and enhance the donation experience. This article provides practical recommendations to support donor well-being and the effectiveness of blood donation programmes worldwide. By highlighting the differences between a modern diet and a diet tailored specifically for blood donors, we aim to emphasise the importance of a nutrient-dense diet for blood donors, which is critical for effective recovery and overall health maintenance. It is important to understand and incorporate different nutrient-dense foods that influence iron absorption for optimal health in blood donors. Donor health is also influenced by regular physical activity and psycho-emotional well-being. The ‘healthy donor effect’ and its implications for maintaining higher standards of donor health are explored in this review. This article highlights the fact that the diet of blood donors is influenced by gender, as men and women have different nutritional needs and physiological responses, particularly with regard to iron levels and recovery. The importance of enhancing or inhibiting iron absorption provides valuable evidence for food fortification as a cost-effective solution to reduce iron deficiency in blood donors.Introduction
Blood donations play a vital role in medical treatment and emergency care, providing essential components for surgery, trauma care, cancer treatment, and chronic diseases [1]. Blood donors are an essential part of the healthcare system. The blood component quality requires adherence to the specified properties and characteristics of the blood component delivered to the consumer. Strict adherence to approved standards and procedures is required at all technological stages and provides a guarantee of the quality of blood components and their finished products [2], from the planning of the donation to the receipt of the finished products and their storage conditions. An adequate blood supply is crucial for the treatment of many diseases, such as anaemia or cancer [3].
Nutrition plays a critical role in the health and performance of blood donors [4]. Proper nutrition before and after blood donation has a direct impact on blood quality, donor well-being, and speed of recovery. Several key aspects highlight the importance of nutrition for blood donors: blood quality, prevention of weakness and dizziness, faster recovery after blood donation, long-term health of donors, and strengthening of the immune system [5].
The declining pool of potential donors has a negative impact on the volume of blood donated in many countries [6]. It is well known that the decline in the donor pool against the background of the growing demand for blood components and preparations is a pressing issue in modern transfusion medicine, as the number of donors is decreasing, especially in the aftermath of the SARS-CoV-2 pandemic. In Mexico, for example, the number of donors decreased by 22% between April and May 2020, compared to the same months in 2019 [7]. Some studies have identified the following main causes of the decline in the number of blood donors: economic and social problems, declining population health levels, an increase in infectious diseases, a lack of interest on the part of employers in the donation of their employees in the context of the migration of employees from the public sector to private companies, weak promotion of donation and irrational use of the country's donor potential, in particular the limited operation of mandatory social and government programmes that should help to stimulate the blood-forming function and metabolism in the donor's body [6, 8].
It is known that, in the case of significant blood loss as well as the violation of the regulated number of donations during the year and a lack of careful monitoring of metabolic processes in the donor's body, donors may experience disorders of macro- and microelements, amino acid, protein, carbohydrate metabolism, and enzyme systems, which ultimately leads to the formation of deficiency conditions and disease states in regular blood donors [9]. These changes causing donors to withdraw from donation due to haemoglobin levels are mainly described in the literature as a consequence of unregulated donation. The effects of haemoglobin deficiency are related to disturbances in the metabolism of iron and trace elements ensuring adequate haemoglobin synthesis and erythropoiesis and in the functioning of metal-dependent enzyme systems and related metabolic processes [10].
Equally important, but often overlooked, are the psychological and social aspects of giving blood. Donors may feel anxious or stressed about the process of donating, and a lack of guidance on nutrition and recovery can exacerbate these feelings. Providing comprehensive information and support can improve the donor experience and make individuals more likely to return and donate again. Promotion of the benefits of proper nutrition and post-donation care can therefore foster a sense of community and shared responsibility, as donors realise that their contribution is not just a single act but part of a wider ongoing effort to save lives. Addressing these multifaceted aspects of blood donation can improve both donor satisfaction and overall programme effectiveness [8].
Several studies have investigated indicators that characterise iron metabolism in the donor's body and the condition of peripheral blood erythrocytes as a function of donor experience, the effect of collection methods and storage conditions on the quality of fresh frozen plasma, the health status of donors at different stages of plasma donation, the quality of fresh frozen plasma, etc. [11, 12, 13, 14]. Maintenance of donor health requires good nutrition, as blood donors are at increased risk of iron deficiency [15, 16, 17]. Iron deficiency without anaemia and iron deficiency anaemia are potential harms to regular blood donors; therefore, maintaining donor health is no less important than ensuring a safe and continuous blood supply [14]. However, the problems of optimising donation through donor nutrition and its medical and social aspects are not yet fully understood.
Thus, while much emphasis is placed on the act of donating blood, less attention is paid to the preparation and post-donation care, particularly from a nutritional perspective. Ensuring optimal health of blood donors before and after donation is critical for maintenance of a safe and robust blood supply. Such factors as diet, hydration, and general health have a significant impact on the quality of donated blood and the recovery time of donors. A better understanding of these factors can help to mitigate potential adverse effects and encourage more frequent and reliable donations [19].
Using key terms such as “blood donation”, “blood donor nutrition”, “nutrient effects” on “blood donors” and “iron deficiency”, this study aims to explore the relationship between diet and blood donation. The research focuses on the role of appropriate dietary practices in supporting the health of blood donors, addressing concerns such as iron deficiency and the effects of essential nutrients. The ultimate goal is to promote safe and informed blood donation, while allaying common fears associated with the process. The aim of this article is to provide a comprehensive overview of the effect of diet on the health and performance of blood donors addressing their nutritional needs, explaining the underlying molecular mechanisms, and providing practical recommendations to support the well-being of donors and the effectiveness of blood donation programmes worldwide. The review was based on search of the PubMed, Web of Science, Scopus, Google Scholar, Cochrane Library, ScienceDirect databases. Elucidation of the key differences between a modern diet and a diet tailored specifically for blood donors is essential to understanding the purpose of this article. By highlighting these differences, we aim to emphasise the importance of a nutrient-dense diet for blood donors, which is critical for effective recovery and overall health maintenance. This explanation forms the basis of the article and highlights the significant impact of a targeted dietary approach on the well-being of individuals who donate blood on a regular basis.
The objective of this study is to investigate the relationship between nutrition and blood donation, with a particular focus on the nutritional needs of blood donors and the impact of nutrients on their health. Key aspects include addressing iron deficiency and providing evidence-based dietary recommendations to support safe and informed blood donation practices. In this way, we aim to encourage more people to donate blood while allaying concerns about potential risks.
Health, nutrition, and blood quality
The importance of blood donation cannot be overstated, as it is a critical component of healthcare systems worldwide, ensuring that life-saving blood and blood products are available to patients in need. Despite its importance, the specific nutritional needs of blood donors remain an under-researched area, often overlooked in both scientific research and public health discussions [13]. Blood donors face unique nutritional challenges, particularly in replenishing iron and other essential nutrients lost during donation. Understanding the impact of nutrition on donor recovery and performance is critical to optimising donor health and ensuring the sustainability of blood donation programmes [18]. This knowledge gap requires focused attention and thorough research to provide clear guidance and support to donors.
The main differences between a modern diet and a diet tailored specifically for blood donors lie in their goals and nutrient focus, as shown in Fig. 1. Modern diets often prioritise convenience, taste, and availability, often leading to high consumption of processed foods and sugary drinks that are low in essential nutrients. In contrast, a blood donor's diet is carefully designed to support overall health and facilitate recovery after donation by emphasising nutrient-rich foods. This includes increased intake of iron-rich foods, such as lean meats and leafy vegetables, to replenish blood stores and vitamins A, C, and E to support cell repair and antioxidant protection [17]. In addition, a blood donor's diet should ensure adequate intake of essential minerals, such as zinc and copper, which are critical for immune function and red blood cell production, as well as proper hydration and avoidance of processed foods to ensure the diet provides all necessary nutrients without excess harmful additives [14, 20].
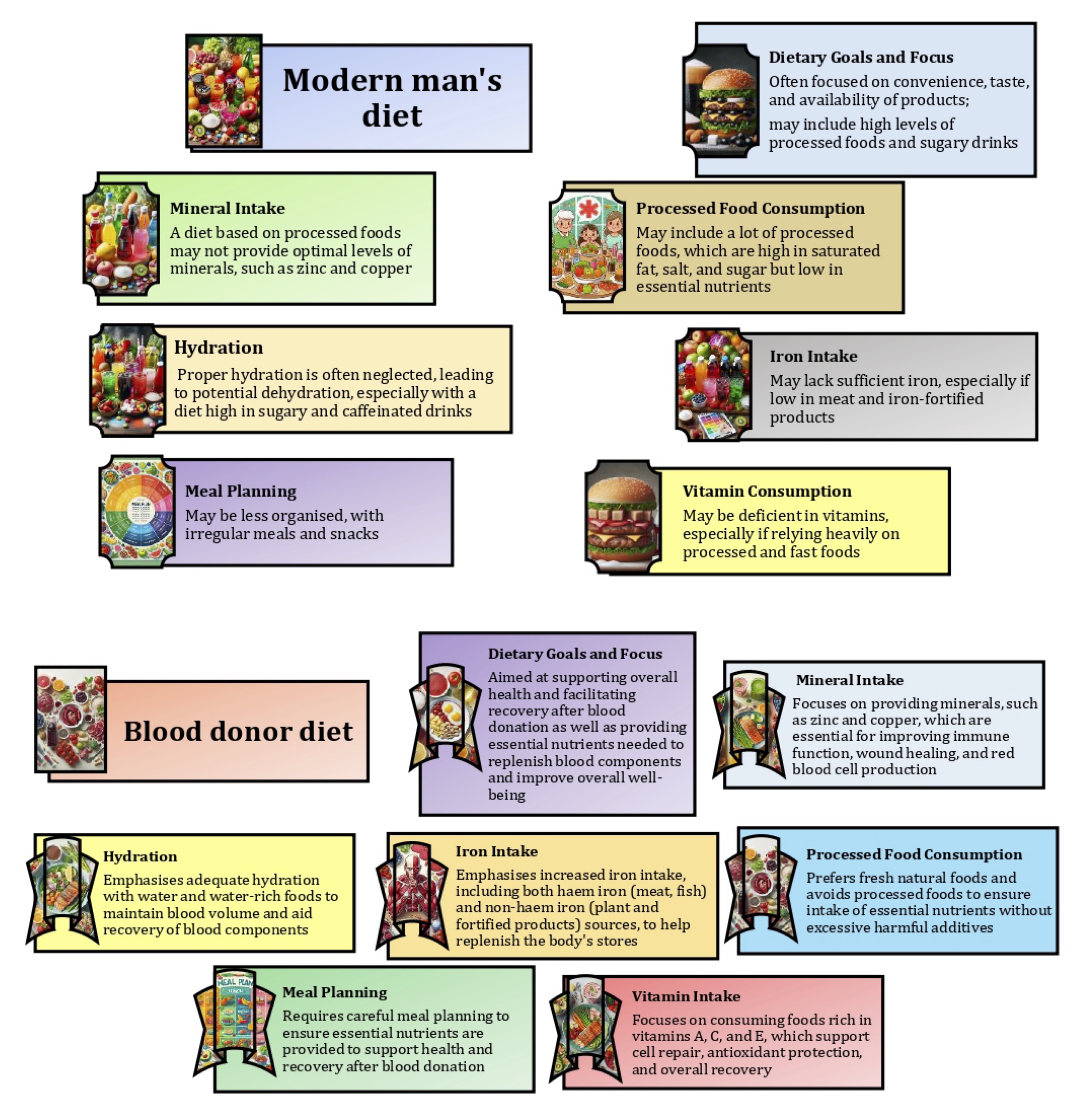
Fig. 1: Main differences between a modern diet and a diet for blood donors.
The physiological requirement for iron is relatively low, but in the event of acute or chronic blood loss, iron requirements increase. Iron is essential for normal erythropoiesis and enters the bone marrow in the following ways: during the destruction of red blood cells, from the depot, and with food and water. The daily diet of an adult must contain 12-15 mg of iron for normal erythropoiesis and for diets providing 18 mg iron/2000 kcal, according to the Recommended Dietary Allowances for iron and energy for women aged 23-50 years [21]. Reference Daily Allowances (RDAs), i.e. the average daily intake of nutrients for healthy people to meet their dietary needs, have been established by the Food and Nutrition Board, Institute of Medicine (IOM) as shown [22, 23]. In addition, an average intake (AI) for iron has been established for infants from birth to 6 months of age, corresponding to the average iron intake of healthy breastfed infants. According to the IOM, the AI for infants aged 0-6 months and the RDA for infants aged 7-13 months are 0.27 mg/day and 11 mg/day, respectively. For children aged 1-3 years, 4-8 years, and 9-13 years, the values are 7 mg/day, 10 mg/day, and 8 mg/day respectively. For adolescents (14-18 years), the RDA is different for boys and girls. While the RDA for male adolescents is 11 mg/day, the RDA for female adolescents is 15 mg/day. For adults aged 19 and over, the RDA is 8 mg/day for men and 18 mg/day for women. In addition, the RDA is 10 mg/day for lactating women under 18 years of age and 9 mg/day for women over 18 years of age. The RDA for vegetarians also varies. The RDA for a vegetarian male is 14 mg/day; in turn, the RDAs for vegetarian females aged 14-18, 19-50, and over 51 are 26 mg/day, 14 mg/day, and 33 mg/day respectively. Iron deficiency occurs when the body's iron needs are not met, which may lead to health problems [22].
Normally, iron in the body is in a dynamic equilibrium [24]. Of the approximately 10 mg of dietary iron, 1-2 mg is absorbed by duodenal enterocytes. In the circulation, iron is bound to transferrin (about 3 mg), which safely transports it to the bone marrow for haemoglobin synthesis. About two-thirds of the body's iron is found as haemoglobin in red blood cells (1800 mg) and erythrocyte precursors in the bone marrow (300 mg), while 10-15% is found in myoglobin and various enzymes. Iron is stored in parenchymal liver cells (about 1000 mg). Reticuloendothelial macrophages temporarily store iron recovered from senescent erythrocytes (600 mg) in a readily available form [24, 25]. Erythropoietin, produced by the kidneys, regulates iron absorption in the duodenum and erythropoiesis. In the human body, iron is a component of almost 70 important enzymes [26]. As suggested by Waldvogel-Abramowski et al. [27], the dietary intake of iron in a typical European diet is about 15 mg, of which only about 10% is absorbed, as absorption occurs via complex mechanisms mainly in appropriate regions of the gastrointestinal tract, especially in the duodenum and proximal jejunum [28].
There are two types of iron found in food, including haem iron and non-haem iron [29]. Haem iron is found exclusively in animal products, such as meat, fish, and poultry, whereas non-haem iron is found in fruits, vegetables, dried beans, nuts, and cereal products [30]. Haem iron is more efficiently absorbed from the gut than non-haem iron [31, 32]. Strict control of dietary iron intake is essential to maintain iron levels within the normal range and reduce the risk of iron deficiency. Iron absorption has been shown to be 25-30% with innards, 7-9% with green leafy vegetables, 4% with cereals, and 2% with dried pulses, suggesting that the type of food or other dietary factors may affect iron bioavailability [33]. For example, ascorbic acid is a known dietary factor that improves iron bioavailability [34], whereas calcium, polyphenols, phytates, and oxalates reduce iron absorption in the gut [35, 36]. Therefore, the type of foods in the diet should be considered to maintain iron balance in the body (Fig.1).
Consideration of the nutritional adequacy of iron highlights the importance of assessment methods, particularly the usefulness of the 'bioavailable nutrient density' approach to different meals [37]. The total body iron content is approximately 4.5-5 g, with 75-80% of iron in haemoglobin, which carries oxygen to tissues and 5-10% of iron in the blood. 5-10% is part of myoglobin, and 1% is part of respiratory enzymes that catalyse respiratory processes in cells and tissues. 20-25% of iron in the body is reserve iron. The physiological loss of iron in urine, sweat, faeces, hair, and nails is about 1 mg/day, independent of age and sex. In women with a normal menstrual cycle lasting 3-4 days, iron losses are about 15 mg (30-50 ml of blood). In hyperpolymenorrhoea (up to 50-250 ml of blood), iron losses increase significantly. During pregnancy, labour, and lactation, up to 1700-1800 mg of iron is lost [38].
The body loses approximately 1-2 mg of iron per day through sloughing of enterocytes and skin, bleeding, and parasitic invasion. As there is no active mechanism for iron excretion, iron homeostasis requires daily intestinal absorption of 1-2 mg of iron. This requirement increases in physiological conditions, such as growth, pregnancy, and menstruation. Meanwhile, about 25 mg of iron is processed daily by macrophages of the reticuloendothelial system through phagocytosis of ageing red blood cells. This means that the majority of human iron homeostasis depends on iron recycling [27, 39]. Therefore, when there is an increased demand for the element, its deficiency is compensated by depleting reserves and then transporting resources. In celiac disease (gluten enteropathy), iron and B12 deficiency anaemia is also found in combination with malabsorption of other nutrients [40].
Diet has an impact on the quality of donor's blood. A balanced diet rich in essential nutrients, such as iron, vitamin B12, and folic acid, is essential for the production of healthy red blood cells. Iron is needed for the production of haemoglobin, which carries oxygen in the blood. Iron deficiency can lead to anaemia, which can disqualify a donor [17].
Psychological and emotional aspects also play an important role in the long-term health of blood donors. Regular blood donation can provide psychological benefits, such as a sense of altruism and satisfaction from helping others [41]. However, some people may experience stress or anxiety associated with the blood donation process. It is important to monitor the diet for nutrients that may affect mood, such as omega-3 fatty acids, B vitamins, magnesium, and tryptophan. These nutrients play a role in the production of neurotransmitters, e.g. serotonin, which regulate mood and stress levels. Social support from family, friends, and medical staff as well as an appropriate diet can make a significant difference to the emotional well-being of donors and ensure that they remain physically and emotionally healthy in the long term [6, 8, 12].
Therefore, maintaining the health and nutritional status of donors is critical to ensuring the quality of donated blood [42]. Regular health checks and monitoring of medical parameters of donors help to eliminate potential risks associated with blood-borne diseases, which has a direct impact on the safety of recipients [43]. A balanced diet rich in vitamins and minerals supports the body's recovery after blood donation and ensures that donors can provide high quality blood [44]. Iron is particularly important, as it is essential for the production of haemoglobin, i.e. the protein in red blood cells that carries oxygen. Adequate iron levels prevent anaemia and ensure that donors remain healthy and able to donate regularly [14]. Education on healthy lifestyles and medical support for donors are essential to maintain their ability to donate regularly, which is fundamental to an effective blood donation system [42].
Prevention of adverse physiological reactions in blood donors
Blood donation is important for ensuring that life-saving blood and blood products are available to patients in need. Giving blood can be stressful for some people, especially first-time donors or those with a fear of needles. The rapid withdrawal of blood during donation can cause a temporary reduction in blood volume, which can trigger a vasovagal response [45]. A vasovagal reaction (VVR) is a common type of fainting or syncope that can occur in blood donors as a result of an autonomic nervous system reflex [46, 47]. VVR is a general feeling of discomfort and weakness with anxiety, dizziness, and nausea, which can progress to loss of consciousness [48]. This reaction can cause a sudden drop in the heart rate and blood pressure, leading to reduced blood flow to the brain and fainting [50]. Before fainting, a donor may experience light-headedness or dizziness, nausea, sweating, blurred vision or spots in front of the eyes, ringing in the ears, a feeling of warmth or flushing, weakness, paleness, and slow or irregular pulse [45, 50]. Non-hypotensive hypovolemia, such as that observed with blood donation, leads to a reflex readjustment of cardiac autonomic tone [49]. VVRs are generally benign, but they can be distressing for the donor and require the attention of medical staff.
VVR is fairly common, with rates varying between 2-5% among donors, depending on such factors as age, gender, and donation history [45, 50]. The risk factors for VVR include young age (under 30), first-time donor, low body weight, and anxiety, all of which increase the likelihood of experiencing a VVR [48]. Most vasovagal reactions are self-limiting and resolve without long-term consequences. However, donors who experience a severe reaction may be reluctant to donate again [45, 50]. Reassurance and education about prevention can help them to feel more comfortable returning for future donations.
Frequent blood donations can lead to lower iron levels, which can cause anaemia [51, 52]. The molecular mechanisms underlying the absorption of iron and the process of its recovery by the body after blood donation are complex and not fully understood [53]. Each donation is associated with a significant loss of approximately 200-250 mg of the functional iron pool in the donor's body, representing approximately 30% of the average body iron stores (BIS) in men and nearly 80% in women [51, 54]. It may lead to a high incidence of subclinical iron deficiency in long-term donors, affecting both women and men [14, 52]. Iron is stored in the form of ferritin (easily mobilised reserve) or haemosiderin (difficult to mobilise reserve) [55]. Plasma transport includes transferritin iron and accounts for about 1% of iron in the body [27]. Iron supplied by enterocytes (5%) and released by recycling of old red blood cells with the participation of mononuclear macrophages (95%) is mainly transported to the bone marrow [56].
Excessive iron intake leads to the formation of its specific biochemical form that can generate free radicals, which have been shown to cause complications [57]. Iron overload has been associated with oxidative stress in organs and tissue damage [58, 59, 60]. In this scenario, luminal iron may interfere with the mucosal barrier and its functions or create a harmful environment in the gut, inducing stress in epithelial cells. Some authors have suggested that elevated liver iron levels due to dietary iron overload may be associated with structural changes in the cecal mucosa [61, 62]. A study by Lobo et al. [57] suggests that these changes in the cecal mucosa may result from oxidative stress caused by excess iron in the intestinal lumen. These effects have significant consequences for intestinal absorption and implications for liver iron homeostasis.
Excessive amounts of iron in food or drug therapy over a period of days can lead to reduced iron absorption. This phenomenon, known as 'mucosal blockage', can be observed even with iron depletion. Excess iron can be observed in haematological patients after repeated blood or erythrocyte transfusions. Teams of authors provide a comprehensive review of the mechanisms and implications associated with the phenomenon of reduced iron absorption due to excess iron intake, namely 'mucosal blockage'. Each study covers different aspects of iron metabolism, from molecular regulation to clinical implications and effects on the gut microbiota [63-65].
Modern ferrokinetic studies have shown that, with a standard blood donation of 450 ml, a donor loses approximately 250 mg of iron from the functional iron pool [54, 66]. Given that 1 ml of red blood cells contains 1 mg of iron, it can be calculated that the iron loss from the apheresis methods of red blood cell collection is equal to or greater than that from whole blood donations. It can be argued that a regular blood donor loses between 500 and 1000 mg of iron per year. Due to the physiological characteristics of the female body, female blood donors are more susceptible to iron deficiency [67]. For example, a study by Prados Madrona et al. [68] on the role of women in altruistic blood donation in Huelva, a province in south-western Spain, showed that women are more altruistic than men in donating blood; a higher percentage of donors and first-time donors were observed among women. However, women also have more difficulties donating blood and are more prone to vasovagal reactions, which negatively affect their donor experience.
A recent study by Schreiber et al. [67] showed that regular blood donation affects ferritin levels differently in men and women. In women, frequent donation leads to higher ferritin levels, possibly due to increased iron absorption and the older age of frequent donors. In men, frequent donation leads to lower ferritin levels, indicating a greater depletion of iron stores. Age plays an important role in modulating these effects, with adjustments for age altering the observed differences in ferritin levels. Anaemia indicators suggest that new female donors are more prone to anaemia, while frequent donation appears to reduce this risk, highlighting the body's adaptive response to regular blood loss. In the case of men, anaemia remains rare even among frequent donors, highlighting the importance of understanding gender-specific responses to blood donation for effective donor health management [67].
It is difficult to formulate effective dietary recommendations for blood donors without a full understanding of these mechanisms. To improve the health of donors and ensure that they can continue to donate safely and regularly, it is essential to fill these knowledge gaps. By shedding light on these critical issues, one can improve donor well-being, increase the efficiency of blood donation, and ultimately support the wider healthcare system. Intake of appropriate meals before giving blood helps to prevent weakness and dizziness that may occur during or after donation. Meals rich in complex carbohydrates, protein, and healthy fats stabilise blood glucose levels and provide the energy needed to maintain well-being during the donation process [69].
Therefore, in addressing the challenges of preventing fainting and dizziness in blood donors, it is important to recognise that women have more difficulty donating blood and are more susceptible to vasovagal reactions. This increased susceptibility has a negative impact on their donor experience [63]. Adequate iron levels play a critical role in this context, as iron is essential for the production of haemoglobin. Ensuring that donors, particularly women, have adequate iron levels through diet or supplementation can significantly reduce the risk of vasovagal reactions. Targeted strategies to mitigate these reactions, particularly in female donors, need to be implemented to improve donor retention and the overall donor experience. Improved donor education, proper hydration, and supportive care during and after donation as well as monitoring and management of iron levels can help to reduce the incidence of vasovagal reactions and ensure a more positive experience for all donors.
Iron content and sex hormones
The differences in iron levels between men and women are largely influenced by sex hormones, which regulate iron metabolism through a number of specific mechanisms. These differences can be attributed to the action of oestrogen and testosterone, which have different effects on iron homeostasis. The role of oestrogen in iron metabolism depends mainly on the regulation of oestrogen and hepcidin and on menstrual blood loss [70]. Lehtihet et al. [71] have shown that oestrogen down-regulates the expression of hepcidin, a key hormone produced by the liver that regulates iron homeostasis. Hepcidin controls iron absorption by binding to the iron exporter ferroportin on enterocytes in the gut and macrophages, leading to its degradation and reducing iron export into the bloodstream [72]. Lower hepcidin levels in women, especially premenopausal women, result in increased ferroportin activity and increased absorption of dietary iron and mobilisation of stored iron [73]. On the other hand, the regular menstrual cycle in women leads to periodic blood loss, which significantly affects iron levels. Therefore, oestrogen regulation of hepcidin is crucial to adapt to this regular iron loss by increasing intestinal iron absorption to compensate for the loss [74].
Testosterone stimulates erythropoiesis by increasing erythropoietin production in the kidneys and promoting the differentiation of erythroid precursor cells in the bone marrow [75]. This results in higher haemoglobin levels and red blood cell counts in men, contributing to greater overall iron demand and utilisation [76]. Testosterone also suppresses hepcidin production, similar to oestrogen, but through different signalling pathways [77]. This suppression facilitates increased iron absorption and mobilisation to support higher erythropoietic activity in men. As a result, men generally have higher serum ferritin levels than women [78].
Differences in iron absorption and storage have also been shown to be associated with important sex-related differences in iron metabolism [79, 80]. The efficiency of iron absorption in the gut differs between men and women and is influenced by different levels of sex hormones. The effect of oestrogen in reducing hepcidin and increasing ferroportin activity results in a more dynamic adaptation to iron loss and demand in women [74]. Men maintain a steady state of higher iron absorption efficiency because they have consistently lower hepcidin levels due to testosterone [81]. Ferritin, the primary intracellular iron storage protein, is also subject to sex-specific regulation. The hormonal influence on hepcidin levels affects iron storage capacity in the liver, spleen, and other tissues [82]. Due to their higher erythropoietic activity and steady iron absorption rates, men tend to store more iron in ferritin [83].
A study by Stangerup et al. [66] focused on the menstrual cycle-influenced recovery period to investigate the effects of blood donation on exercise performance in women. Specifically, the study aimed to identify changes in VO2peak, time trial performance, and haematological variables in 18 iron-sufficient women with plasma ferritin > 30 µg/L following a standard 450 mL blood donation. The results showed that the VO2peak and blood haemoglobin levels did not return to baseline until 28 days after donation, whereas the time trial performance recovered within 14 days. This suggests that, while the overall physical performance measured by the time trial can recover relatively quickly, the full recovery of aerobic capacity and haemoglobin levels takes longer, highlighting the need for tailored recovery protocols for female donors, particularly those who menstruate [66].
It was noted that gender-specific recommendations for donor nutrition are essential to address the different physiological needs of male and female donors. Women are at higher risk of iron deficiency due to menstruation and should prioritise iron-rich foods such as lean red meat, beans and fortified cereals, along with vitamin C to enhance iron absorption. Men, while generally having higher baseline iron stores, should still ensure adequate nutrient intake to maintain optimal recovery and overall health after donation.
In summary, the interaction between iron content and sex hormones, such as oestrogen and testosterone, has significant implications for blood donors. Oestrogen in women can lead to lower iron levels due to menstrual blood loss, making female donors more susceptible to iron deficiency and related complications during donation. On the other hand, testosterone in men tends to support higher iron levels, which may explain why men generally have fewer problems with iron deficiency during the donation process. Understanding these hormonal influences is crucial for developing targeted strategies to manage iron levels in donors and ensure that both male and female donors maintain adequate iron stores. This approach will enhance donor safety and improve the overall donation experience, contributing to a more reliable blood supply.
Faster recovery from blood donation
Proper nutrition after giving blood is a key to a speedy recovery [84]. Meals rich in protein support tissue repair, while carbohydrates help to restore energy levels quickly. Hydration is also important to maintain adequate fluid levels in the body, which helps to return to normal more quickly after blood donation [85]. Intake of appropriate meals before giving blood helps to prevent weakness and dizziness that may occur during or after donation. Meals rich in complex carbohydrates, protein, and healthy fats stabilise blood glucose levels and provide the energy needed to feel good while donating [86]. Inclusion of a variety of nutritious foods in the diet means consumption of a wide range of foods that are rich in essential vitamins, minerals, and other nutrients but relatively low in calories and unhealthy additives [87, 88].
For blood donors in particular, this approach ensures a balanced intake of nutrients needed for optimal health and recovery. For example, inclusion of iron-rich foods, such as lean meats, legumes, and dark leafy greens, helps to replenish iron stores, while consumption of fruits and vegetables rich in vitamins A, C, and E supports immune function and protects against oxidative stress [89]. In addition, intake of foods rich in minerals (e.g. zinc and copper), such as nuts, seeds, and whole grains, supports wound healing and red blood cell production [90-92]. By diversifying their food choices and emphasising whole unprocessed foods, individuals can maintain a healthy diet that supports their general well-being as well as the specific needs associated with blood donation. This is illustrated in Fig. 2.
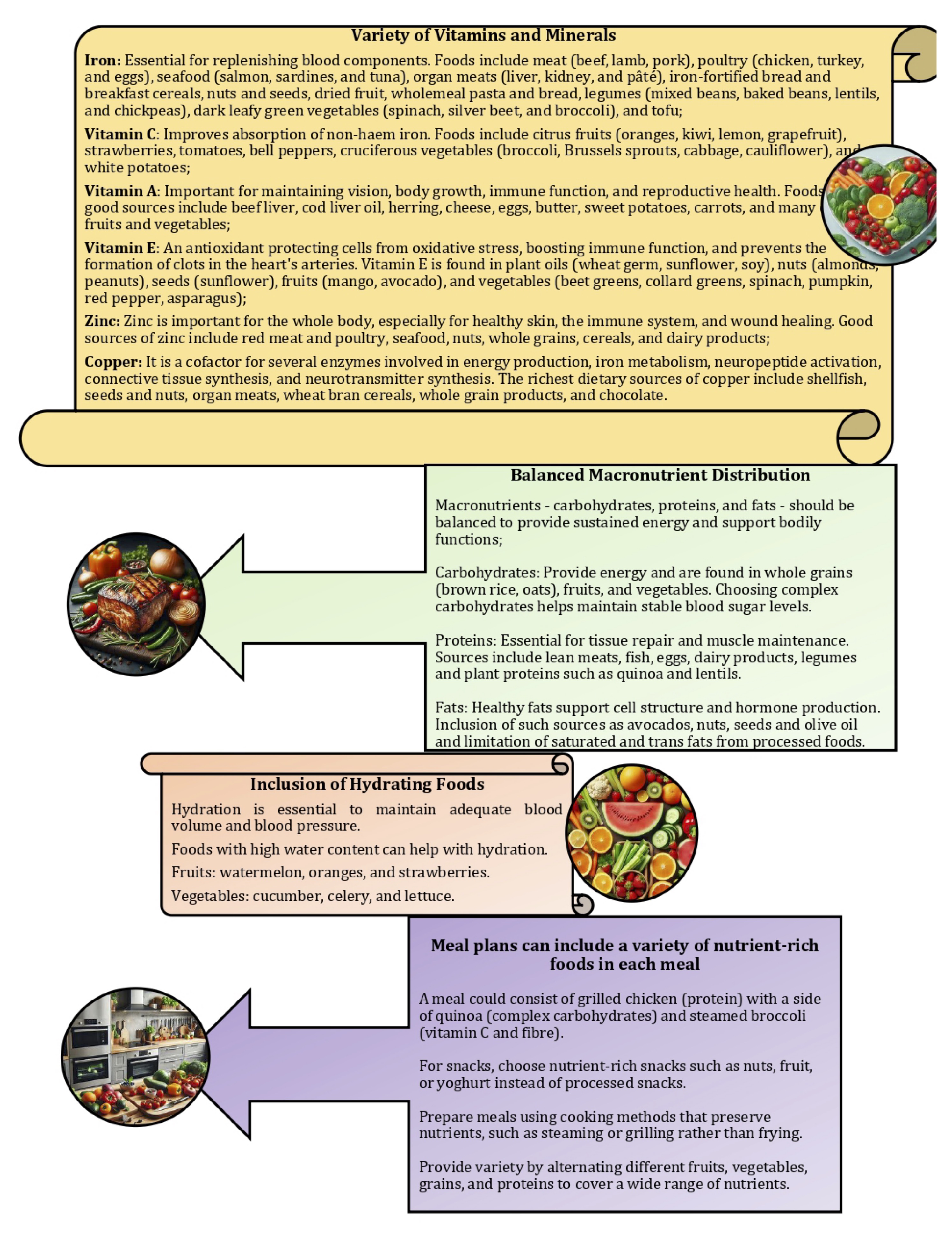
Fig. 2: Planning meals for donors aiming to include a variety of nutritious foods in the diet means inclusion of a wide range of foods that provide essential vitamins, minerals, and other nutrients while being relatively low in empty calories.
A study by Hallberg and Rossander-Hultén [93] revised iron requirements for specific population groups, indicating that adult women require 2.84 mg of absorbed iron per day, while adolescents require 3.21 mg per day. These requirements are based on the 95th percentile, meaning that they meet the needs of 95% of people in these groups. To translate these absorbed iron requirements into dietary requirements, the researchers made six independent estimates of total dietary iron bioavailability and found that the bioavailability of iron in the Swedish, French, and US diets was 14%, 16%, and 16.6%, respectively. By averaging these results, 15% bioavailability was used to represent optimal long-term iron absorption from a typical Western diet. Another study looked at different dietary components that affect iron absorption, including enhancers such as vitamin C and inhibitors such as tannins, calcium, and phytates [36]. This study showed that tannic acid decreased the fasting bioavailability of non-haem iron; however, this effect was not present in the presence of calcium, and no effect of phytic acid or citrus pectin on the fasting bioavailability of non-haem iron was observed in the presence or absence of calcium [36].
Iron absorption occurs primarily in the duodenum and proximal jejunum and involves several mechanisms [94]. Haem iron is absorbed more efficiently than non-haem iron, with non-haem iron requiring reduction from ferric (Fe3+) to ferrous (Fe2+) iron prior to absorption [95]. The hormone hepcidin, produced by the liver, regulates iron absorption by promoting the degradation of ferroportin, i.e. an iron exporter [29]. Based on bioavailability estimates, dietary iron requirements were calculated to meet absorbed iron needs: adult menstruating women need 18.9 mg per day, while menstruating adolescents need 21.4 mg per day. The study by Hallberg and Rossander-Hultén [93] highlights the importance of understanding both physiological iron requirements and the bioavailability of dietary iron, which is crucial for making accurate dietary recommendations, especially for populations at higher risk of iron deficiency, such as menstruating women and adolescents (Fig. 2).
Wiersum-Osselton et al. [96] investigated risk factors for vasovagal reactions and needle-related complications during blood donation, focusing on their impact on donor return. They found that first-time donation is an established risk factor for vasovagal reactions, which in turn negatively affects donor return rates. Interestingly, among first-time donors, women experienced fewer vasovagal reactions than men. Other risk factors in this study showed similar associations in both first-time and repeat donors. Regardless of whether donors were first-time or repeat donors, both vasovagal reactions and needle-related complications led to a decrease in subsequent donation rates, highlighting the importance of addressing these issues to maintain a stable pool of blood donors.
In summary, to facilitate faster recovery after blood donation, it is important to focus on incorporating a variety of nutritious foods into the diet to meet the absorbed iron requirement. Iron-rich foods should be prioritised to efficiently replenish iron stores. Examples of such iron-rich foods include a wide range of options. i.e. red meat (beef, lamb, and pork), poultry (chicken and turkey), fish and seafood (salmon, tuna, sardines, mussels, and clams), pulses (lentils, chickpeas, black beans, kidney beans, and soybeans), tofu, fortified cereals, dark leafy vegetables such as spinach, nuts and seeds (pumpkin seeds, sunflower seeds, and almonds), dried fruits (raisins, apricots, and prunes) and quinoa [97, 98]. In addition, combining iron-rich foods with those high in vitamin C, such as citrus fruits and peppers, can help to improve iron absorption [99]. By incorporating these dietary practices, donors can recover more quickly and maintain their overall health and willingness to donate in the future. This holistic approach not only supports individual donor well-being, but also helps to maintain a reliable and healthy donor pool.
Long-term health of blood donors
Blood donation involves the removal of a significant volume of blood from the body, which contains not only red blood cells but also essential nutrients, such as iron, vitamins, and minerals [100]. Proper nutrition before and after donation is essential to replenish these lost components and maintain donor health. Nutritional mechanisms therefore explain how a balanced diet rich in these essential nutrients supports the rapid recovery of blood donors, ensuring that they remain healthy and able to donate regularly [87]. Regular blood donation requires a healthy lifestyle, including proper nutrition. Healthy eating habits help to maintain appropriate haemoglobin levels and overall good health, allowing regular and safe blood donation [101].
The specifics of studying iron metabolism are crucial for understanding the recommendations for rapid rebuilding of the iron pool in long-term blood donors [100]. This detailed knowledge allows researchers and health professionals to determine the exact mechanisms by which iron is absorbed, transported, stored, and utilised in the body. It is well known that the special role of iron in biological systems is related to its ability to change its oxidation state over a wide range of oxidation-reduction potentials [102, 103]. This can be further modified by co-ordinated ligands. An extremely high level of iron enters the body, passes the rate-limiting absorption step, and becomes saturated. This free iron enters cells in the heart, liver, and brain. By interfering with oxidative phosphorylation, free iron converts ferrous iron to ferric iron, which releases hydrogen ions and increases metabolic acidity. Free iron can also lead to lipid peroxidation, resulting in severe damage to mitochondria, microsomes, and other cellular organelles, involving cellular oxidation and reduction mechanisms and their toxicity to intracellular organelles [104]. The hydrogen free radicals produced by iron attack DNA lead to cell damage, mutation, and malignant transformation, which in turn cause a range of diseases [105].
Living organisms have developed numerous mechanisms to tightly regulate the uptake, storage, utilisation, and export of iron. At the cellular level, the expression and translation of genes encoding proteins that modulate iron uptake, storage, utilisation, and export are regulated by iron regulatory proteins, sensors of intracellular iron levels, and post-transcriptional modifications. At the systemic level, the liver controls body iron levels by producing the peptide hormone hepcidin. Hepcidin reduces the amount of iron entering the bloodstream by blocking the function of ferroportin, the only iron exporter in mammals [106]. Cells have developed metabolic strategies to import and utilise iron safely. Regulation of iron uptake, storage, intracellular trafficking, and utilisation is critical for maintaining cellular iron homeostasis [107].
Iron is known to be involved in the Fenton reaction, which leads to the formation of the highly reactive hydroxyl radical and the formation of dinitrosyl iron complexes (DNICs) [108, 109]. In vivo, the two reactants of the Fenton-like reaction, the ferric citrate complex and H2O2, are readily available [108].The Fenton-like reaction of ferric citrate with H2O2 has been proposed as one of the mechanisms of the labile iron pool that can induce oxidative stress and cause pathological processes in the body [110, 111]. In addition, lysosomes also contain a redox-active iron pool derived from iron-rich macromolecules (ferritin) and cellular organelles (mitochondria) [112].. One of the possible sources of intracellular redox-active iron is the degradation of ferritin in lysosomes during autophagy [113] . The hydrogen peroxide that diffuses into lysosomes can react with the iron species through the Fenton and Fenton-like reactions, resulting in the generation of hydroxyl radicals [110].
Paramagnetic DNICs, discovered over 50 years ago as the first naturally occurring nitric oxide complexes in living organisms, may be a form in which iron ions are removed from most cells. Despite the tight control of iron levels and its storage as complexes with proteins, some iron is present in the cell in the form of a pool of iron labily bound to low molecular weight ligands that can readily undergo a Fenton reaction [114]. The increased production of nitric oxide and other radicals during oxidative stress is accompanied by an increase in the pool of labile iron as a result of its release from iron-sulphur cluster-containing proteins, such as aconitase or Rieske's protein [115]. At the same time, the iron contained in DNIC formed in cells is largely from the labile pool, and iron ions released from ferritin in oxidative stress conditions participate in the synthesis of DNIC with glutathione [116].
A number of molecular mechanisms involving aspects other than iron metabolism are engaged in the long-term health of blood donors. One key factor is blood regeneration, which requires the activation and proliferation of bone marrow stem cells [117]. Haematopoietic stem cells (HSCs) are a group of pluripotent stem cells found in haematopoietic tissues that can differentiate and produce a variety of mature blood cells (red blood cells, macrophages, platelets, lymphocytes, etc.) to maintain the normal physiological functions of living organisms [118]. Erythropoiesis is exquisitely regulated by an oxygen-sensing mechanism that has evolved to maintain the number of red blood cells within a narrow physiological range [119, 120]. Central to this mechanism is erythropoietin (EPO), a cytokine secreted by the kidney in response to low blood oxygen tension [121]. HSCs can also respond to infection or injury by rapidly entering the cell cycle and differentiating, often preferentially along the myeloid lineage [122]. Additional insult-related signals may intervene to regulate the HSC fate in such dynamic conditions. It is known that both mature immune cells and HSCs can be activated either by direct activation of pathogen recognition receptors (PRRs), such as Toll-like receptors (TLRs), or by pro-inflammatory cytokine signalling [123, 124]. In addition, the bone marrow microenvironment, including lining cells, blood vessels, and extracellular matrix components, plays an important role in maintaining HSC homeostasis and function, which is critical for the health of blood donors [125].
Another important aspect of donor functioning is the immune system. Regular blood donation can stress the immune system [126]. Blood donors need to maintain a healthy immune system to prevent infections and other diseases, because T and B lymphocytes, which play a key role in the immune response, need to be constantly regenerated [127]. Such factors as interleukins (e.g. IL-2, IL-6, IL-7) are thought to be critical for the proliferation and differentiation of these cells [128]. In a study by Al-Hazimi [126], the stress on the immune system induced by the donation of 500 mL of blood was measured. The decrease in CD4+ cells (T helpers) and the increase in CD8+ cells (T suppressors) led to an imbalance, resulting in an overall decrease in the CD4/CD8 ratio. The CD8 lymphocyte subpopulation also has suppressive activity, which may have contributed to the decrease in CD4 cells. An increase in CD56+ (NK) cells was observed, which may indicate an improvement in the immune system. This may have been a result of a positive psychological effect that counteracts the stress. The stress induced by the blood donation also led to a significant increase in IgG, IgA, and IgM. Increased adrenaline levels may have triggered this increase in immunoglobulins to prepare the body's defence system to fight foreign invaders, such as bacteria, that may attack in stressful conditions [126].
Redox balance and oxidative stress within the donor cells are also important. Antioxidants, such as glutathione, and antioxidant enzymes, e.g. superoxide dismutase, catalase, glutathione-related enzymes such as glutathione reductase, and glutathione peroxidase, play a key role in protecting cells from oxidative damage caused by free radicals [129]. Dietary non-enzymatic antioxidants play an equally important role. Important non-enzymatic antioxidants for blood donors include vitamin C, vitamin E, glutathione, α-lipoic acid, coenzyme Q10, uric acid, and α-carboxylic acid [130]. In excess, free radicals can be harmful. However, they have physiological roles in cell differentiation, immune cell activation, metabolic adaptation, and autophagy. It has therefore been proposed that their presence is not harmful and may contribute to maintaining health [131]. While an ideal antioxidant blend should reduce free radical levels, it is important to ensure that optimal levels of free radicals are present [130]. Therefore, monitoring and supporting these processes can help to maintain the long-term health of blood donors, minimise the risk of complications, and support overall immunity.
In addition to blood regeneration and immune system function, another important aspect of the health of long-term blood donors is cardiovascular health [132, 133]. Regular blood donation affects the volume of circulating blood, which in turn can affect haemodynamic parameters, such as blood pressure and heart rate [134, 135]. To maintain haemodynamic balance, the body activates compensatory mechanisms, such as changes in the activity of the renin-angiotensin-aldosterone (RAA) system and the secretion of antidiuretic hormone (ADH) as shown [136, 137]. It is also important to monitor the diet to ensure adequate intake of electrolytes, such as sodium and potassium, and nutrients that support cardiovascular health. A diet rich in vegetables, fruit, whole grains, healthy fats, and protein can help to maintain circulatory stability and healthy blood pressure [138]. The key to maintaining circulatory stability is the regulation of fluid and electrolyte volume [139]. Long-term blood donors should also maintain a healthy diet and regular physical activity to support cardiovascular health [140].
Importance of proper nutrition for blood donors
Good nutrition is the foundation of good health for blood donors. It not only ensures the quality of the blood donated, but also supports the donor's well-being and rapid recovery [141]. It is therefore important that donors pay attention to their diet and ensure that their body receives all the essential nutrients. At a molecular level, the absorption and metabolism of iron – a critical element in blood production – is highly dependent on dietary intake and overall nutritional status [103].
Lipemia, characterised by the presence of "opaque cloudy plasma", poses significant challenges to the production and use of blood components at the time of donation [142]. The presence of lipemic blood, resulting from various physiological and paraphysiological causes, metabolic disorders, common diseases, and certain medications, can complicate several aspects of transfusion medicine [143]. These difficulties include epidemiological, technical, analytical, clinical, and economic concerns. Due to the interference of lipemia with laboratory tests and the potential safety issues associated with the use of hypertriglyceridemic blood, most national and international guidelines discourage the use of lipemic donations for the production of blood components [144, 145]. While chemical or mechanical methods can reduce some assay interferences, the suitability of lipemic blood for clinical use remains unresolved. The prevalence of lipemic donations is generally between 0.31% and 0.35%, although some reports suggest it may be as high as 13% [146]. These two different types of donor plasma are shown in Fig. 3, with good quality plasma on the right and lipemic plasma on the left.
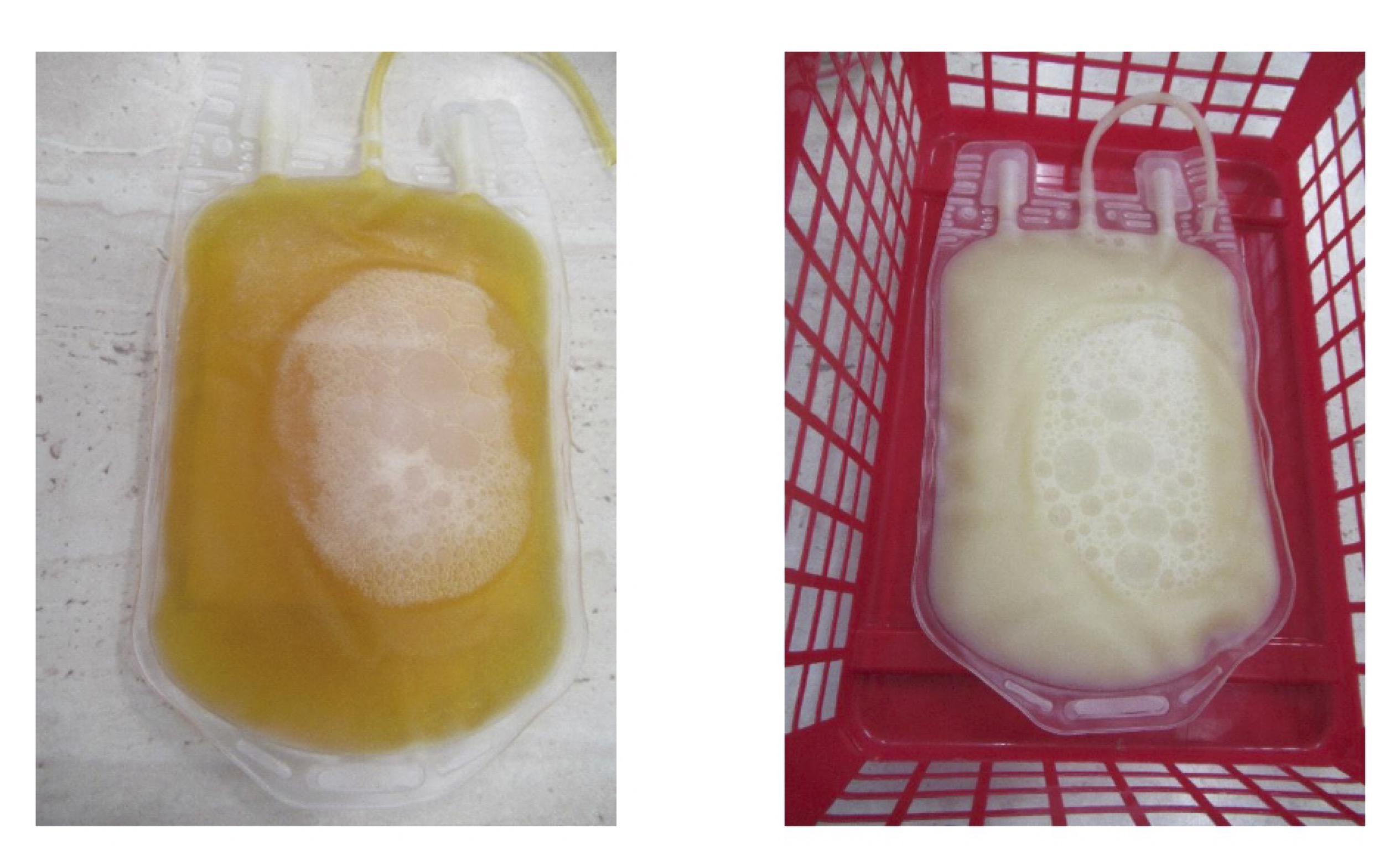
Fig. 3: On the left, the plasma is clear and transparent, indicating normal lipid levels in the blood. This is the desired state of plasma, allowing accurate laboratory testing and safe production of blood components. On the right, the plasma shows lipemia. The plasma is cloudy and milky in appearance, indicating high levels of lipids, particularly triglycerides, in the blood. This condition can occur for a number of reasons, including a high fat diet, metabolic disorders, or the use of certain medications. Before donating blood, donors should be instructed on proper dietary practices, especially avoiding fatty foods, to prevent lipemia. Regular monitoring of lipid levels and treatment of metabolic disorders may also help to reduce the incidence of lipemia. In transfusion medicine, it is important to develop and implement uniform guidelines for handling lipemic donations to ensure the safety and efficacy of blood and its components. Photo by Natalia Kurhaluk.
In addition, lipemia can sometimes lead to haemolysis of red blood cells, although this is less common. The presence of high levels of lipids in the plasma can cause physical changes or stress to red blood cells, potentially leading to their destruction. This haemolysis can further complicate the use of lipemic blood to produce safe and effective blood components [147]. Another study reported that there was no significant difference in haemolysis between erythrocytes from lipemic and non-lipemic whole blood donations when stored in saline-adenine-glucose-mannitol. The proportion of erythrocytes from lipemic donations with higher haemolysis was greater than in controls, so there was a weak correlation between erythrocyte haemolysis and plasma triglycerides [148]. The transfusion medicine community must work towards universal agreement and harmonised policies to effectively address the challenges posed by lipemic donations. This includes the formulation of clear guidelines and the adoption of consistent practices to ensure the safety and reliability of blood components derived from donations.
Iron bioavailability is the proportion of dietary iron absorbed in the gut and used for physiological functions, particularly haematopoiesis [29]. Absorption, i.e. the availability of iron for intestinal absorption, is sometimes used as a synonym for bioavailability, but good absorption is only one of the prerequisites for good bioavailability [149]. Bioavailability depends on the degree of absorption and the incorporation of absorbed iron into erythropoiesis [29]. Iron fortification of foods is considered the most cost-effective approach to reducing the prevalence of iron deficiency [150, 151]. The bioavailability of iron-based salts in the human body is in the following order: ferrous sulphate, ferrous lactate, ferrous fumarate, ferrous succinate, ferrous glycine sulphate, ferrous glutamate, ferrous gluconate > ferrous citrate, ferrous tartrate, ferrous pyrophosphate > ferric citrate, ferric sulphate [152]. Bioavailability can be improved by chelating iron using NaFeEDTA or ferrous glycinate, which improves luminal iron solubility [152].
There are two types of iron that can be found in food, i.e. haem iron and non-haem iron. Haem iron is only found in animal products such as meat, fish, and poultry. Non-haem iron is found in fruit, vegetables, dried beans, nuts, cereals, and meat [153]. The body absorbs haem iron more efficiently, but absorption of non-haem iron can be improved by intake of foods rich in iron absorption enhancers [29]. Ascorbic acid (vitamin C), folic acid, citric acid, peptides rich in the amino acid cysteine, and vitamin A are iron absorption enhancers [154]. Non-haem iron absorption can be enhanced by carotenes, retinoids, alcohol (by increasing gastric acid secretion, which promotes the valence state], and citric, tartaric, and malic acids [155]. Ferritin and transferrin play key roles in iron storage and transport, while hepcidin regulates iron balance by inhibiting absorption when iron levels are sufficient [156]. A healthy diet ensures that these molecular mechanisms function properly, supporting efficient blood production and recovery after donation; hence, a nutritious, well-balanced diet that includes iron-rich foods and foods rich in iron absorption enhancers is recommended for blood donors.
Furthermore, the long-term health of blood donors depends on a number of other mechanisms that involve aspects other than iron metabolism [157]. In blood donors, non-enzymatic antioxidants, including vitamin C (ascorbic acid), vitamin E (tocopherols and tocotrienols), glutathione, lipoic acid, coenzyme Q10 (ubiquinone), uronic acid, and α-carboxylic acid, play a key role in protecting cells from oxidative stress [158, 159]. Vitamin C is a powerful water-soluble antioxidant that protects cells from free radical damage, helps to regenerate other antioxidants such as lipid-soluble vitamin E, protects cell membranes from oxidative damage, and helps to protect lipids from oxidation [160]. Glutathione, a tripeptide consisting of glutamate, cysteine, and glycine, plays a key role in cellular detoxification and free radical scavenging as well as the regeneration of other antioxidants, such as vitamins C and E [161-163]. Lipoic acid is a potent antioxidant that acts in both oxidised and reduced forms to support the regeneration of other antioxidants and protect cells from oxidative stress, promoting energy metabolism and detoxification [164]. Coenzyme Q10, which is a fat-soluble compound, plays a key role in energy production in mitochondria and in protecting cells from oxidative damage [165]. Uronic acid, a product of glucose metabolism, acts as an antioxidant by neutralising reactive oxygen species [166]. Alpha-carboxylic acid, found in several fruits and vegetables, helps to protect DNA and other biomolecules from oxidative damage [167]. A diet rich in these antioxidants may help to protect the health of blood donors by supporting their resistance to oxidative stress and improving their overall body condition.
Blood donors should focus on a balanced diet rich in tryptophan, tyrosine, omega-3 fatty acids, magnesium, and antioxidants in the days before donation, as this can help to prepare the body and mind, reduce anxiety, and improve mood [168-171]. A healthy breakfast or lunch prior to the donation should include protein (lean meat, cheese, and yoghurt), complex carbohydrates (bread, cereals, and fruit), and iron-rich foods (red meat, fish, poultry, beans, and raisins). After the donation, continuous consumption of a nutritious diet will support recovery and maintain emotional well-being, with hydration essential to prevent fatigue and maintain mental clarity [172]. Dietary recommendations before and after blood donation are shown in Figures 4 and 5.

Fig. 4: Dietary recommendations after blood donation consist of the following elements: iron-rich foods, foods rich in protein and complex carbohydrates, foods rich in vitamin C with iron-rich meals improving the ability to absorb of iron, adequate hydration products, and products to avoid after blood donation: alcohol and caffeinated beverages.
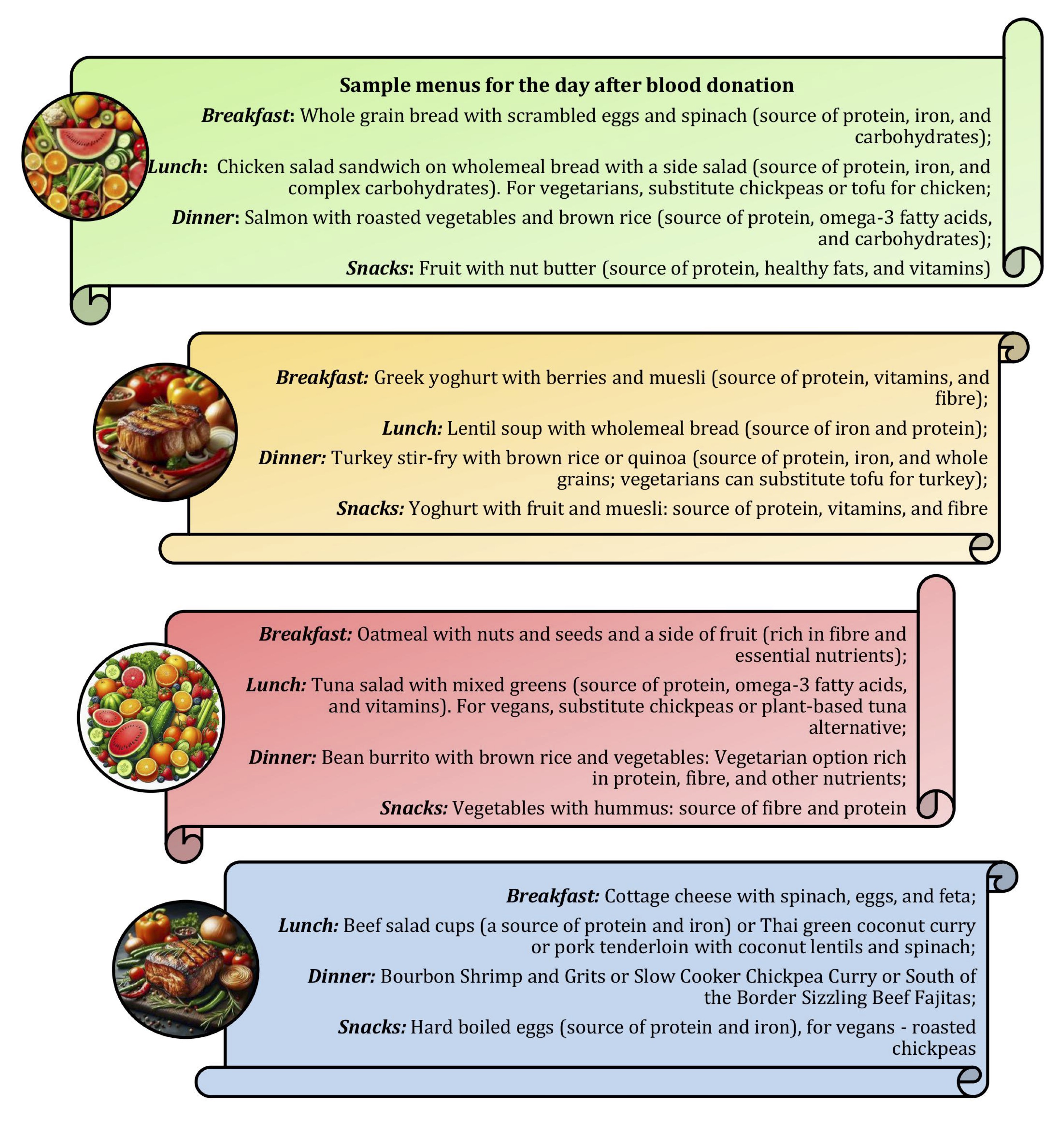
Fig. 5: Sample menus for the day after blood donation.
Thus, proper nutrition of blood donors is therefore of paramount importance (Figs 1 and 2). Educating donors about eating a balanced diet, avoiding fatty foods prior to donation, and staying well hydrated can help to reduce the incidence of lipemia. Good dietary habits improve not only the quality of blood donations but also the overall health of donors and the efficiency of the blood donation process. Addressing lipemia requires a multifaceted approach that includes understanding its causes, managing its impact on blood component production, and emphasising the importance of proper donor nutrition. Establishing clear guidelines and harmonising policies worldwide can help to mitigate the challenges posed by lipemic donations, ensure the safety and reliability of blood components, and promote healthier donation practices.
Iron metabolism
Iron in the body is conventionally divided into several categories that encompass the different forms and functions of this element [173]. These include functional iron, transport iron, stored iron, and free pool iron. Each of these forms of iron has specific functions and is essential for the proper functioning of the body. Maintaining a balance between the different forms of iron is essential for good health, and both iron deficiency and iron excess can lead to serious health problems. Iron is a vital micronutrient playing an essential role in many biological processes such as oxygen transport, DNA synthesis, enzyme function, and others. Its diverse functions lead to different classifications based on a number of criteria [174].
The main pools of iron in the body consist of haem (cellular), non-haem (extracellular), and iron stores (depots), as shown in Fig. 6. Haem (cellular) iron makes up a significant proportion (70-75%) of iron in the body. It is involved in internal iron metabolism and is part of haemoglobin, myoglobin, enzymes (cytochromes, catalase, peroxidase, NADH dehydrogenase), and metalloproteins (aconitase, etc.). Non-haem extracellular iron consists of free plasma iron and iron-binding serum proteins (transferrin, lactoferrin) involved in iron transport [175, 176]. Iron stores (depots) are present in the body in the form of two protein compounds, ferritin and haemosiderin, which are mainly deposited in the liver, spleen, and muscles. They are incorporated into metabolism in the event of cellular iron deficiency [177, 178].
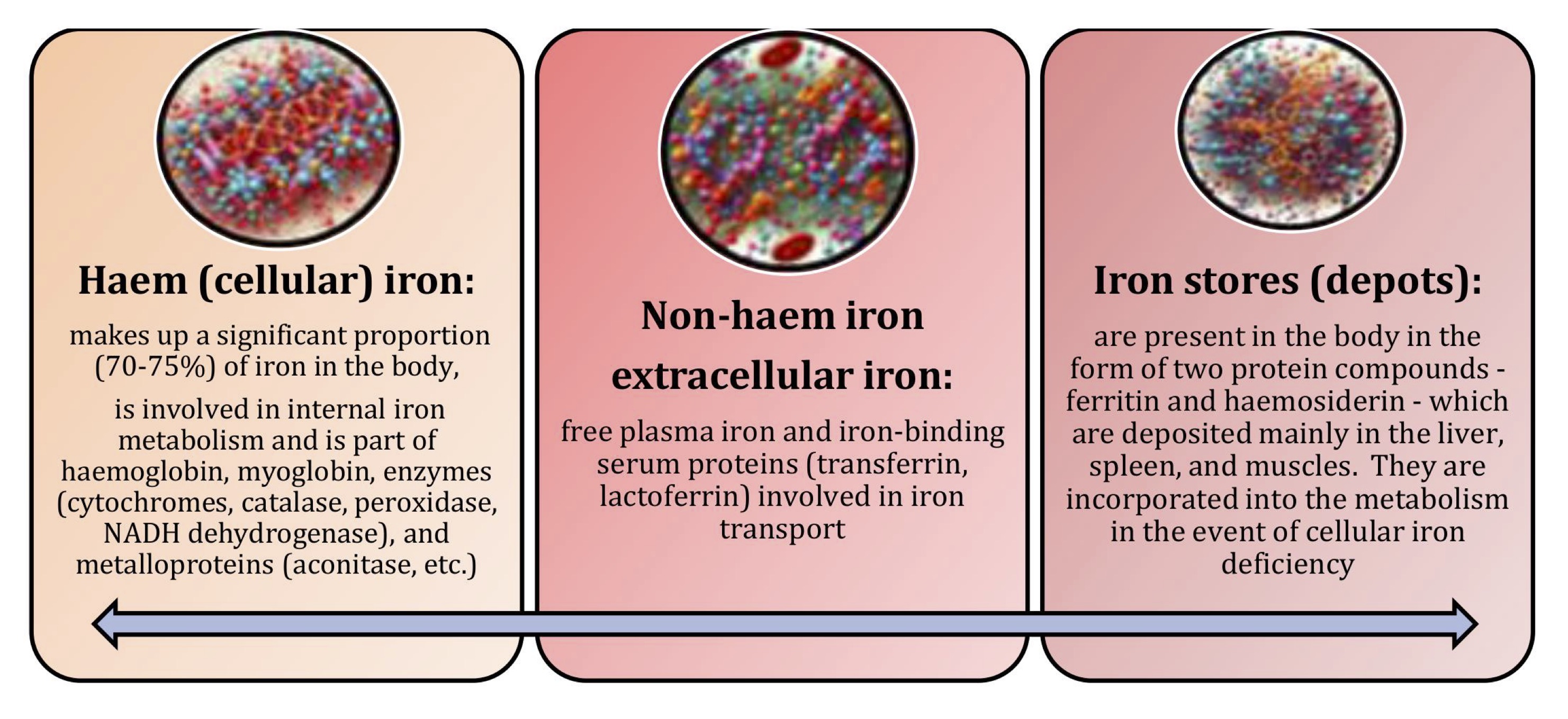
Fig. 6: Main pools of iron in the body.
As a result of these diverse functions, its role as functional iron, transport iron, storage iron, and free pool iron may vary depending on the research and clinical context (Fig. 7). Functional iron is directly involved in biological functions as part of key proteins and enzymes. Functional iron is associated with haemoglobin – a protein found in red blood cells responsible for transporting oxygen from lungs to tissues and carbon dioxide from tissues to lungs, and myoglobin – a protein found in muscles that stores oxygen and facilitates its rapid release during exercise [179].
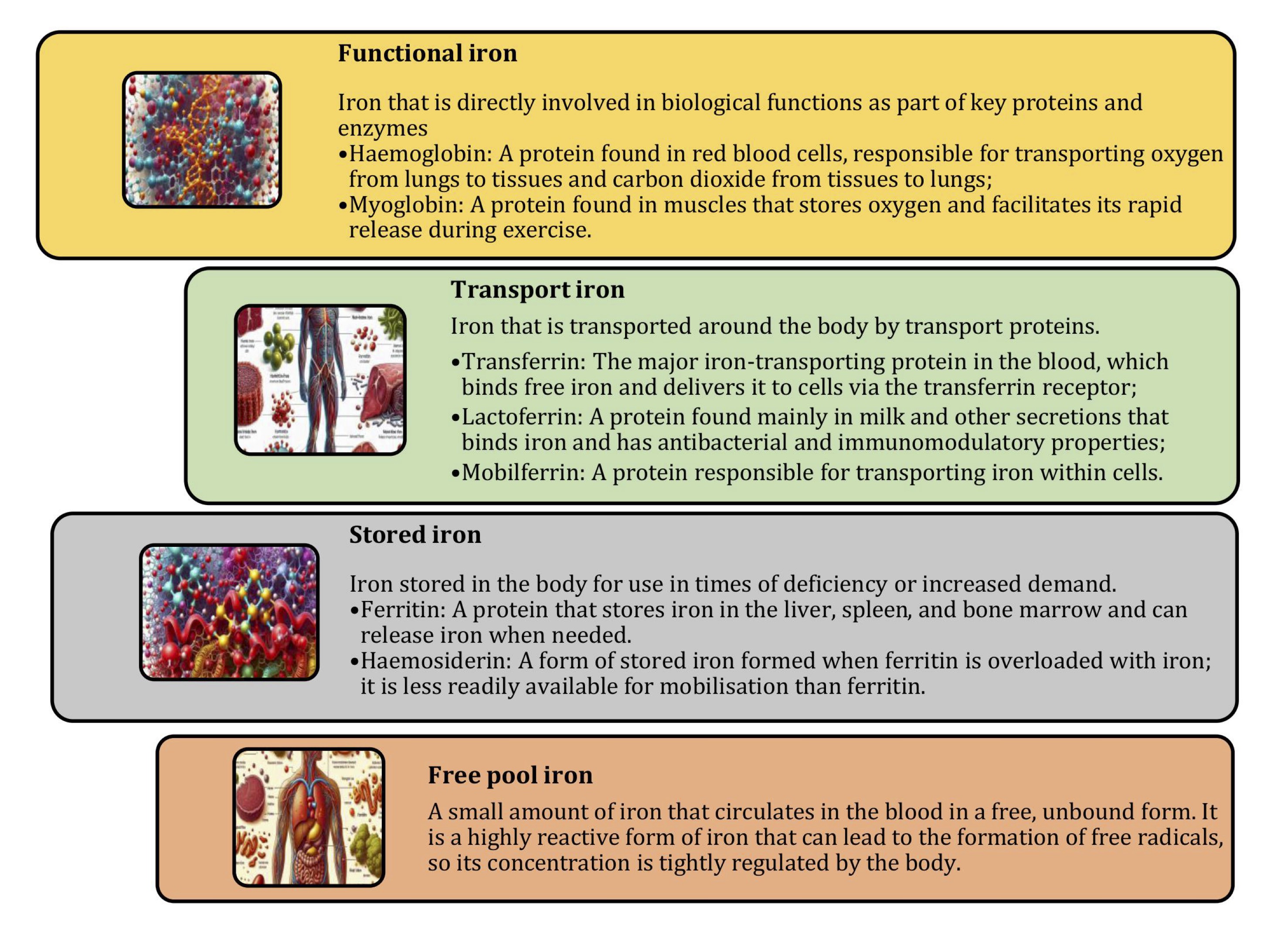
Fig. 7: Different forms of iron in the body.
Iron is transported throughout the body by transport proteins, such as transferrin, lactoferrin, and mobilferrin [181, 182, 185]. Transferrin is the major iron-transporting protein in the blood, binding free iron and delivering it to cells via the transferrin receptor [186]. Lactoferrin, i.e. a protein responsible for transporting iron within cells found mainly in milk and other secretions, binds iron and has antibacterial and immunomodulatory properties [183]. Stored iron is iron deposited in the body for use in times of deficiency or increased demand. The main forms of stored iron are ferritin and haemosiderin [184]. Ferritin acts as a protein that stores iron in the liver, spleen, and bone marrow and can release iron when needed, and haemosiderin is a form of stored iron formed when ferritin is overloaded with this element; it is less readily available for mobilisation than ferritin [55]. A small amount of iron circulates in the blood in a free, unbound form as free pool iron [180]. It is a highly reactive form of iron that can lead to the formation of free radicals; therefore, its concentration is tightly regulated by the body [103].
Coordination of iron homeostasis processes
Coordination of iron metabolism, particularly in donors, ensures balanced iron homeostasis, which is essential for proper cellular function [64]. Adequate iron supply supports critical biological activities, such as oxygen transport and energy production, while regulatory mechanisms prevent the potential toxicity of excess iron [103]. Research into these molecular mechanisms provides a deeper understanding of iron physiology and pathophysiology and may lead to better treatments for disorders associated with iron imbalance. Iron homeostasis relies on a complex network of proteins that manage the uptake, transport, storage, and regulation of iron in the body. At the forefront of iron absorption are divalent metal transporter 1 (DMT1) and ferroportin 1 (FPN1). DMT1, located on the apical membrane of intestinal enterocytes, is responsible for importing non-haem iron from the diet into enterocytes. Once inside these cells, iron can be exported into the bloodstream by FPN1, which is located on the basolateral membrane. This process allows entrance of iron to the circulation and its delivery to various tissues [187, 188].
Plasma transferrin carries iron around the blood, binding it so that it does not react with other molecules and cause damage. Transferrin transports iron to various tissues and cells where it binds to the transferrin receptor 1 (TfR1) on cell membranes. This binding facilitates endocytosis of the transferrin-iron complex into the cell. In the acidic environment of the endosome, iron is released from transferrin and enters the cytoplasm via DMT1 on the endosomal membrane. This iron can then be used in various metabolic processes, such as haemoglobin synthesis, or stored in cytosolic ferritin to prevent toxicity [189].
The regulation of iron homeostasis is primarily controlled by hepcidin, a peptide hormone produced by the liver [190]. Hepcidin binds to FPN1, causing it to be internalised and degraded, thereby reducing the export of iron from enterocytes and macrophages into the bloodstream. This regulatory mechanism ensures that dietary iron absorption and iron release from stores are tightly controlled to meet the body's needs without causing iron overload. In parallel, intracellular iron regulatory proteins (IRPs) modulate the expression of DMT1, TfR1, ferritin, and FPN1 by binding to iron responsive elements (IREs) in their mRNAs, thereby adjusting protein levels in response to intracellular iron concentrations [191-193].
Iron deficiency occurs when the amount of iron required by the body cannot be met due to a number of physiological consequences, including blood loss and limited dietary intake [190]. Hypoxic and sideropenic syndromes are the main clinical manifestations of iron deficiency anaemia [196]. The hypoxic syndrome has been shown to include symptoms common to all anaemias: pallor, paleness, palpitations, tinnitus, headache, and weakness [197]. Sideropenic syndrome manifestations include taste perversion, dry skin, nail changes, hair loss, angular stomatitis, burning tongue, and dyspeptic syndrome. The diversity of clinical manifestations of iron deficiency is due to the wide range of metabolic abnormalities resulting from the dysfunction of iron-containing and iron-dependent enzymes [103, 198]. Dietary interventions are the most appropriate way to improve iron status and can act as an alternative to conventional treatment [194]. The clinical manifestations of iron deficiency anaemia are shown in Fig. 8.
Less well known clinical manifestations of iron deficiency include neurotic reactions and neurasthenia, decreased muscle performance and general exercise tolerance, and disturbances in myocardial metabolic processes, peripheral circulation (decreased peripheral resistance and venous tone, decreased functional reserves of arterioles), and microcirculation. In the long-term course of iron deficiency anaemia, patients gradually develop myocardiodystrophy and sympathicotonia in the autonomic regulation of cardiac activity [13, 100, 199, 200]. In studies of iron deficiency anaemia, the main clinical manifestations of iron deficiency are lesions of the gastrointestinal tract, manifested as chronic gastritis and syndromes of intestinal absorption disorders [201]. At the same time, the decrease in secretion and acid production in chronic gastritis is considered to be a consequence rather than a cause of iron deficiency, and is explained by dysregenerative processes in the gastric mucosa [202]. It is thought that iron deficiency in the intestinal wall may lead to increased absorption and accumulation of toxic concentrations of iron antagonist metals in the body [203, 204].The disorders of anti-infectious immunity in patients with iron deficiency anaemia reported in the literature are complex in origin, involving inhibition of pathogenic microorganisms that require iron for their own growth and reproduction as well as disorders of cellular mechanisms of resistance to infection due to a decrease in the microbicidal activity of granulocytes and impaired proliferation of lymphocytes [205-207].
Iron compounds, nutrients, and food iron fortification
Iron bioavailability is the proportion of dietary iron absorbed in the gut and used for physiological functions, particularly haematopoiesis [29]. Absorption, i.e. the availability of iron for intestinal absorption, is sometimes used as a synonym for bioavailability, but adequate absorption is only one of the prerequisites for good bioavailability [208, 209]. Iron bioavailability depends on the degree of absorption and the incorporation of absorbed iron into erythropoiesis [210]. Food fortification with iron is considered the most cost-effective approach to reducing the prevalence of iron deficiency [150].
All iron compounds approved for use in foods can be divided into several groups: inorganic salts, organic acid salts, and chelates. The following iron compounds are approved for food fortification: ferrous gluconate, ferrous bisglycinate, ferrous carbonate, ferrous sulphate, ferrous lactate, ferrous fumarate, ferrous citrate, ferrous diphosphate (pyrophosphate), elemental iron (carbonyl + electrolytic + hydrogen reduced), ferric citric ammonium (ferric ammonium citrate), ferric orthophosphate, ferric succinate, ferric saccharate, ferric amino acid complexes, ferric sodium complex of ethylenediaminetetraacetic acid, and sodium ferric diphosphate [211, 212].
The interaction of iron and macronutrients in foods can cause oxidation of product components (lipids), resulting in organoleptic changes (off-flavours). Iron can also cause adverse colour changes by reacting with micronutrients (polyphenolic compounds found in tea, coffee, chocolate, and many fruits) [213-214]. The use of iron pyrophosphate, elemental electrolyte iron, and encapsulated iron, unlike other forms, does not affect the organoleptic performance of the product and does not cause gastrointestinal upset [215, 216]. Water-soluble iron salts have a higher bioavailability but also a greater ability to cause unacceptable changes in product properties [150]. This is because iron ions in solution have a distinct metallic taste; iron can form unacceptable coloured complexes with polyphenolic compounds and oxidise fats in lipid-containing products, such as wheat flour and whole or powdered whole milk [29, 150].
Several studies have looked at the relationship between iron and macronutrients in food [217-219]. The presence of sufficient amounts of iron absorption enhancers [ascorbic acid, fish, poultry, and meat, as found in most developed countries) overcomes the inhibition of iron absorption by even large amounts of tea [33]. Iron absorption may be a problem in individuals with low intakes of haem iron, low intakes of enhancing factors, and/or high intakes of inhibitors. Although depletion of iron stores increases iron absorption, this effect is not sufficient to compensate for inhibited absorption in such an inadequate dietary situation [29]. Studies by Andrews et al. [220] and Jaramillo et al. [36] have shown that calcium, phytic acid, polyphenols, and dietary fibre are major inhibitors of iron absorption and may be present in excess in some diets, thereby altering or modifying iron nutritional status. In addition, phytic acid combined with calcium is a potent inhibitor of iron absorption [220, 221]. Pectin, with or without calcium, was found to slightly reduce iron absorption. Tannic acid showed unexpected behaviour, inducing an increase in iron absorption despite its low ability to dialyse Fe [220].
For people at risk of iron deficiency, the study by Ma et al. [222] recommends increasing the intake of haem iron (this form of dietary iron, found in meat, fish, and poultry, is little influenced by other dietary factors in terms of its absorption). The transport of haem iron across intestinal enterocytes can be reduced by the small amounts of polyphenolic compounds present in food. However, the inhibitory effects of dietary polyphenols on haem iron absorption [223] can be counteracted by ascorbic acid and can be avoided by reducing polyphenol consumption and taking ascorbic acid at the same time [222]. Another recommendation is to increase ascorbic acid intake with meals and to fortify foods with iron. Recommendations for tea consumption (if in a critical group) include drinking tea between meals rather than during meals and consuming ascorbic acid and/or meat, fish, and poultry at the same time [222].
Ferrous sulphate, ferrous gluconate, ferrous fumarate, ferrous pyrophosphate, sodium ferrous ethylenediaminetetraacetic acid (NaFeEDTA), ferrous bisglycinate, and elemental iron powder are the most commonly used forms to fortify foods with iron [211, 224]. Ferrous sulphate and gluconate are soluble in water and gastric juice. Ferrous fumarate is poorly soluble in water but dissolves completely in gastric juice during digestion and is thought to have the same bioavailability as ferrous sulphate [225]. Ethylenediaminetetraacetic acid and bisglycinate are iron chelates with comparable absorption to that of ferrous sulphate in the absence of iron absorption inhibitors [225, 226]. As iron bisglycinate chelate is absorbed unchanged, there is no contact of free iron not only with the gastric mucosa but also with foods that inhibit iron absorption (dairy products, tea, coffee, etc.) [227]. This means that chelated iron can be used independently of dietary intake. Another important advantage of ferrous bisglycinate chelate is its higher bioavailability - almost four times higher than that of ferrous sulphate [228, 229]. This can be explained by the presence of two absorption pathways and binding to two types of receptors. The first type of receptor, DMT1, located in the duodenum, is for iron salts [230]. The second type of receptor, PEPT1, located throughout the small intestine, is designed to bind peptides. The presence of the amino acid glycine in the iron bisglycinate chelate allows it to bind to this type of receptor as well. As a result, the absorption of this compound is significantly increased [227].
It is known that iron is absorbed in the intestine in both ionic and complex forms as well as via the paracellular route. Fe3+ iron ions must first be reduced to Fe2+ by cytochrome b reductase or other reductases at the brush border membrane [231, 232] and by food components acting as reducing agents before being transported into enterocytes by the divalent metal transporter 1 [233]. Iron ions can form chelate complexes with other molecules, which are taken up by endocytosis and importers [232, 234]. It has been concluded that haem and iron bisglycinate have similar absorption properties [235, 236].
For this reason, food fortification with iron is considered the most cost-effective approach to reducing the prevalence of iron deficiency in blood donors [237]. Because of its cost-effectiveness, this strategy can be widely applied to at-risk populations, leading to significant improvements in public health. However, when implementing food fortification programmes, it is important to consider issues related to the dietary inhibitors of iron absorption [30]. These inhibitors can have a significant impact on the effectiveness of iron fortification; hence, addressing them is critical for ensuring that blood donors receive adequate levels of iron [29, 150, 210]. Addressing these factors can significantly reduce the rates of iron deficiency among blood donors, particularly in regions with high incidence of anaemia, with long-term health and economic benefits [237].
Key nutrients and their impact on blood donor well-being
Iron as main meal component
In
healthy individuals, approximately 80% of absorbed iron is used for
haemoglobin synthesis, where iron provides a specific binding site
for oxygen in the haem moiety of haemoglobin in erythrocytes [103,
238, 239]. A whole blood donation results in the loss of
approximately 225-250 mg of iron [240, 241], which can lead to iron
depletion and a subsequent decline in haemoglobin levels if donations
are frequent. Therefore, dietary iron intake is particularly
important for blood donors to maintain iron homeostasis and
haemoglobin levels as shown in Fig. 6-8. As suggested by Skolmowska
and Głąbska [242], adult men and postmenopausal women should
consume 10-11 mg/day of iron. Female blood donors have a much higher
risk of iron deficiency anaemia than men due to menstrual blood loss.
Iron loss in both women and men leads to iron deficiency and
subsequent anaemia if the element is not replenished [243, 244]. The
level of iron in the donor's body depends not only on the frequency
of blood donation but also on many other factors, such as
physiological factors, diet, or lifestyle [244]. It has been shown
that age, body mass index (BMI), and alcohol or meat consumption are
positively associated with iron levels in the body [245, 246], while
age, gender, frequency of blood donation, and differences in
lifestyle of donors can influence the level of haemoglobin in the
blood [241].
Iron
has been shown to be present in such animal products as beef, turkey
(especially dark meat), chicken, lamb, pork, and fish [29]. Beef
contains more total iron (2.2 mg/100 g) than veal (1.5 mg/100 g),
lamb (1.5 mg/100 g), pork chops (1.0 mg/100 g), and chicken breast
(0.9 mg/100 g). The iron content in meat from the same animal varies
in different muscle groups; the iron content in red meat chicken legs
(1.8 mg/100 g) is twice that of white meat chicken breast; duck meat
contains 1.2 mg of iron/100 g, while duck breast has high iron
content of 4.5 mg/100 g. Most minced beef comes from older dairy
cows, which means it has high content of haem iron. Of the total iron
content, haem iron accounts for 83% in beef, 63% in pork chops, and
33-44% in chicken [247]. Giblets and blood products such as black
pudding contain high levels of haem and non-haem iron and meat
factors. For example, the total iron content in pork offal is as
follows: liver 13.4 mg/100 g, liver paté 5.6 mg/100 g, heart 6.0
mg/100 g, kidney 3.3 mg/100 g, and black pudding 16.2 mg/100 g. The
total iron content in lean fish is 0.2 mg/100 g in cod, 0.1 mg/100 g
in flounder, and 0.8 mg/100 g in sea bass. The content of this
element in fatty fish is 0.2 mg/100 g in farmed salmon, 0.8 mg/100 g
in mackerel, 0.7-1.3 mg/100 g in herring, and 1.6 mg/100 g in tuna.
Cod fillet contains no haem iron, whereas 30-40% of the iron in other
fish is haem iron. Fish flesh also contains meat factors; therefore,
the iron contained therein has relatively good bioavailability [247].
Significant
variations in iron content have been observed between different
natural dietary sources, both animal and plant (Fig. 2). A study by
Briguglio et al. [248] summarised the main natural sources of dietary
iron and highlighted their potential contribution to human nutrition.
Among animal foods, organ meats, especially liver, are particularly
rich in iron. The presence of the non-haem-enhancing meat protein
factor (MPF) may improve the
absorption of this iron, as
red meat is a relatively rich source of highly bioavailable haem
iron, providing about 1.1-1.3 mg per 100 g, compared to chicken and
fish, which provide about 0.1-0.9 mg per 100 g, while red meat
contains higher levels of poorly bioavailable non-haem iron [249].
Raw veal and other mammalian livers have an impressive iron level of
20 mg per 100 g. This high concentration makes liver one of the most
potent sources of dietary iron available. Chicken egg yolk and
various types of raw fish both contain around 5 mg of iron per 100 g,
making a significant but smaller contribution than liver. Raw meats,
such as veal and beef, provide about 4 mg of iron per 100 g, which is
substantial but still considerably less than organ meats. In
contrast, cow's milk, a commonly consumed animal product, contains a
negligible amount of iron, i.e. merely 0.2 mg per 100 g [248, 249]
and shown in Figs 4-8.
In
contrast, most dietary iron is in the form of non-haem iron, which is
found in whole grain breads and pasta, whole grains, seeds, nuts,
tofu, peanuts, kidney beans, lentils, oats, chickpeas, peas,
couscous, dried apricots, almonds, spring greens (spinach, kale), and
dried fruits (raisins, apricots, and prunes) as shown [97, 98]. Plant
foods are also valuable sources of iron, although the bioavailability
of non-haem iron from these sources can vary [248]. Dried common
oregano is a top contender among plant foods, providing 18 mg of iron
per 100 g. Bitter cocoa powder, often used in various culinary
applications, provides 14.3 mg of iron per 100 g. Arabica coffee
powder follows with 12 mg of iron per 100 g, highlighting its
potential contribution to dietary iron intake, particularly in
populations with high coffee consumption. Dried pulses such as
lentils and beans are well known for their iron content, providing
around 9 mg per 100 g. These foods are important sources of iron in
many vegetarian and vegan diets. Similarly, wheat bran and soya
flour, both dried, provide about 8 mg of iron per 100 g. Nuts,
including walnuts, almonds, and pistachios, contain around 7 mg of
iron per 100 g, making them a valuable addition to an iron-rich diet.
Edible mushrooms, while not as rich in iron, still contribute between
1 and 2 mg per 100 g, and red wine provides a modest 0.9 to 1.1 mg of
iron per 100 g, which can add up if consumed regularly [248, 249].
The
absorption of non-haem iron is less efficient and more variable, with
only 1-10% being absorbed [250], and is influenced by many dietary
factors [153] and shown in Figs 7 and 8. Dietary iron is absorbed in
the proximal small intestine. Men typically absorb about 1 mg/day,
which corresponds to their basal losses mainly from the
gastrointestinal tract. Premenopausal women absorb more, about
1.3-1.5 mg/day, due to additional menstrual losses [251]. The body's
absorption capacity increases in response to iron deficiency,
reaching an average maximum of 4-5 mg/day in highly active blood
donors, although the average absorption rate is more commonly 2-3
mg/day.51 Maintaining adequate iron levels is essential for blood
donors. This is due to the significant iron loss associated with
donation and the importance of both haem and non-haem iron sources in
the diet [100].
It
has been suggested that a higher intake of non-haem iron is
associated with lower haemoglobin (Hb) levels, independent of haem
iron intake. This is due to the presence of phytate and
polyphenol-rich foods and beverages (such as legumes, cereals, and
coffee), which inhibit the absorption of non-haem iron [103, 241].
Data show that blood donors with higher intakes of haem iron and
lower intakes of non-haem iron tend to have higher Hb levels, a
relationship mediated by higher ferritin levels [241]. The main
sources of dietary iron are meat, poultry, and fish [29]. Haem iron
from haemoglobin and myoglobin accounts for 40% of the total iron in
animal foods and is much more efficiently absorbed (15-40%). In
contrast, non-haem iron makes up all the iron in plant foods [252].
To effectively replenish iron stores and prevent iron deficiency
anaemia, iron-rich products should be included in the diet of blood
donors [14].
Vegetarians
are at greater risk of anaemia than non-vegetarians because their
diets lack or contain lower levels of highly bioavailable haem iron,
and they often struggle to maintain haemoglobin levels required for
repeated blood donations [253, 254]. In addition, certain compounds
in plant foods inhibit the absorption of non-haem iron. To improve
iron bioavailability, vegetarians are advised to increase their
intake of vitamin C and other organic acids [242].
Thus,
the literature highlights the importance of inclusion of both animal
and plant foods in dietary planning and emphasises the diversity of
natural dietary sources of iron [14]. Research into the
bioavailability and absorption of iron from these diverse sources has
informed and refined dietary recommendations and guidelines,
increasing their effectiveness in preventing and treating iron
deficiency. Organ meats, especially liver, have been identified as
particularly rich sources of iron, while various plant foods, such as
oregano and cocoa powder, also contribute significantly [29].
Understanding the iron content in these foods is important,
particularly for those at risk of iron deficiency or requiring higher
iron intakes, such as blood donors, pregnant women, and athletes.
These findings highlight the need for careful dietary planning to
ensure adequate iron intake from a variety of sources.
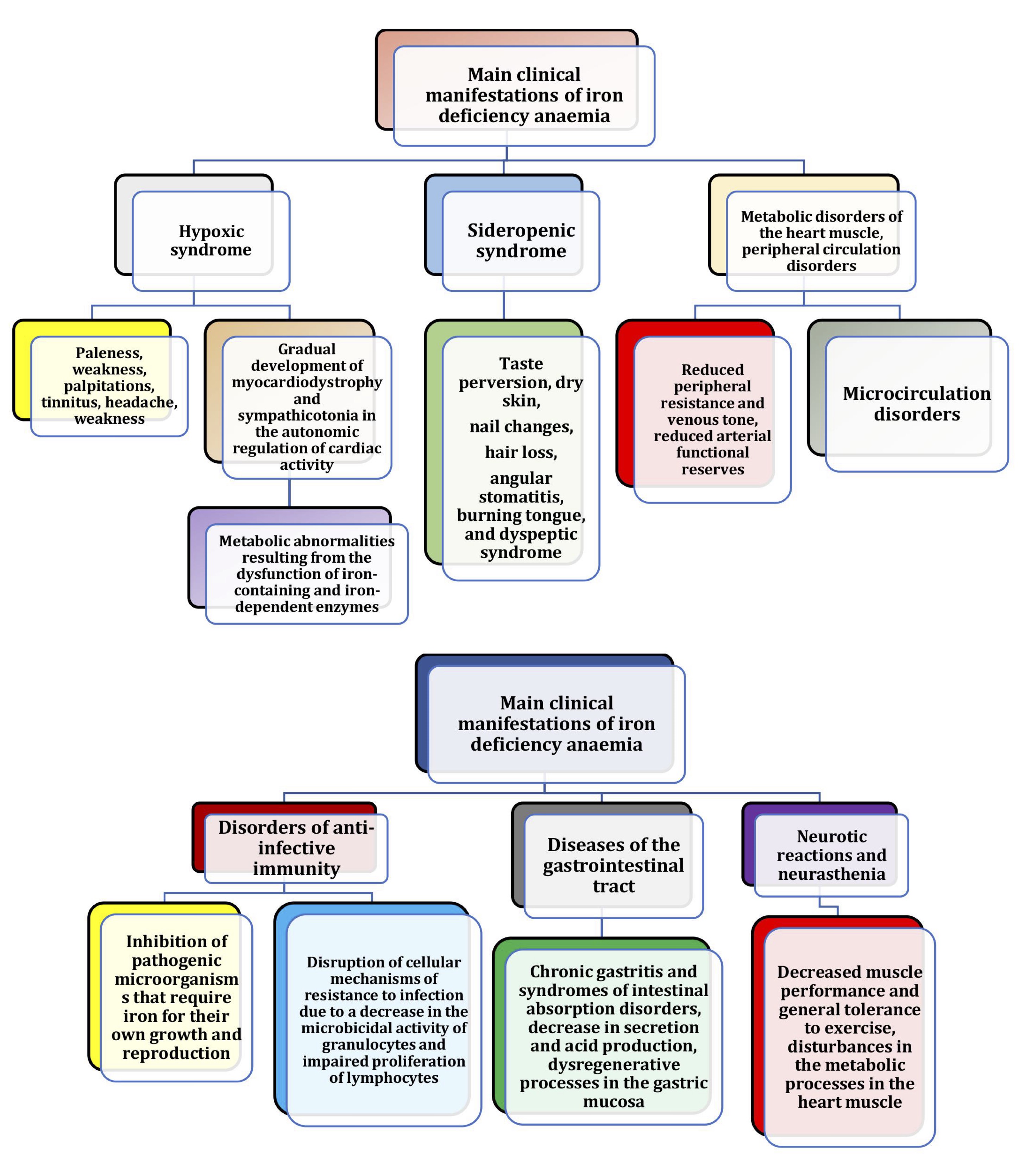
Fig. 8: Clinical manifestations of iron deficiency anaemia.
Influencers and inhibitors of iron absorption
Iron
absorption is known to be influenced by the presence of various
dietary facilitators and inhibitors [238, 255, 256]. By understanding
the iron absorption pathways and their regulation, more effective
dietary strategies and interventions can be developed to combat iron
deficiency, especially in vulnerable populations [257]. These
mechanisms highlight the importance of dietary sources of both haem
and non-haem iron in maintaining adequate iron levels, particularly
in populations at risk of iron deficiency, such as blood donors.
Enhancing the bioavailability of non-haem iron through dietary
strategies, such as consumption of ascorbic acid alongside iron-rich
foods, may mitigate the inhibitory effects of such compounds as
polyphenols, phytates, and other dietary inhibitors [29, 225, 258,
259].
In
the context of dietary iron, the distinction between haem and
non-haem iron is crucial because of their different absorption
mechanisms and efficiencies. Haem iron, found mainly in animal
products, is absorbed more efficiently than non-haem iron, although
the exact mechanism of its uptake by intestinal enterocytes is not
well understood [242]. Non-haem iron found in plant foods is
transported across the apical membrane of intestinal enterocytes by
divalent metal ion transporter 1 (DMT1) and exported into the
bloodstream via ferroportin 1 (FPN1) [259]. Once in the bloodstream,
newly absorbed iron binds to plasma transferrin and is distributed to
various sites in the body, particularly the erythroid bone marrow,
which has high iron requirements [39]. Transferrin-bound iron binds
to transferrin receptor 1 on the surface of most somatic cells and,
after endocytosis, iron enters the cytoplasm via DMT1 in the
endosomal membrane [39]. The bioavailability of dietary iron also
depends on the balance between the
inhibitors and enhancers of
iron absorption [64]. Dietary components affect iron availability
through chemical reactions in the stomach and small intestine lumen,
as shown in Fig. 9.
The
amount of iron absorbed from a food or meal by an individual is
determined by physiological variables, such as body iron status,
combined with the modulating effects of dietary inhibitors and
enhancers. The major dietary enhancers of iron absorption include
vitamin C (ascorbic acid), meat, poultry, fish, and alcohol. In
contrast, the
inhibitors include tannins
(found in tea and coffee), calcium and dairy products, polyphenols,
phytate, animal proteins (from milk and eggs), and other
micronutrients, such as zinc and copper [29, 238].
The
analysis and summary of dietary sources of L-ascorbic acid, as
reported in various studies [256, 258], highlight its important role
in enhancing iron absorption from meals. Ascorbic acid enhances iron
absorption by reducing Fe³⁺ to the more soluble Fe²⁺, which is
necessary for transport into mucosal cells [248]. Ascorbic acid also
binds iron, preventing it from forming complexes with phytate or
tannins that would otherwise make it unavailable to the divalent
metal transporter. An increase in iron absorption is directly
proportional to the amount of ascorbic acid consumed, within a range
of 25-1000 mg [260]. This reducing property of ascorbic acid is one
reason why fruit and fruit juices increase dietary iron absorption
[234, 255]. Vitamin C has a beneficial effect only when taken with
meals [261]. Alcohol has also been positively associated with iron
absorption. It increases the permeability of the intestinal mucosa to
iron and, through the relatively high iron content in certain wines
and beers, contributes to iron absorption [262, 263].
As
reported by Briguglio et al. [248], the dietary sources with the
highest levels of L-ascorbic acid (vitamin C) vary significantly
between fruits and vegetables. Among fruits, raspberries lead with
198 mg of vitamin C per 100 g, followed by kiwi with 141 mg per 100
g, lemons with 129 mg per 100 g, and oranges with 50 mg per 100 g.
Among vegetables, peppers are particularly rich in vitamin C with 584
mg per 100 g, followed by cabbage with 348 mg per 100 g and onions
and garlic with 183 mg per 100 g [248]. These findings highlight the
importance of inclusion of a variety of fruits and vegetables in the
diet to ensure adequate vitamin C intake. In particular, raspberries
and peppers stand out as remarkable sources, making them excellent
choices for increasing daily vitamin C levels. Overall, the diverse
range of vitamin C-rich foods highlights the potential of dietary
planning to improve the nutritional status and overall health of
blood donors.
In
contrast to influencers, some inhibitors reduce dietary iron
absorption. The main dietary inhibitors of iron absorption are phytic
acid, polyphenolic compounds, phosphates, soy protein products, and
various dietary fibres [29, 225]. Phytic acid binds to minerals and
renders them unavailable due to its chelating properties. It is found
in particularly high concentrations in cereals (wheat, maize, rice)
and legume seeds (beans, lentils, and soya), mainly in the bran of
cereals [264]. The content of phytic acid in oilseeds, such as soya
beans, sesame seeds, sunflower seeds, linseed, and rapeseed, varies
from about 1.0 to 5.4% (dw). Maximum phytic acid content of 10.7% has
been reported in soya bean concentrates [265]. Nuts, such as walnuts,
almonds, cashews, etc., with phytic acid contents ranging from about
0.1 to 9.4% [266] are another group of phytate-rich foods. The phytic
acid content in rice bran was found to be 2.15% [267]. It has been
reported that phytic acid inhibits iron absorption. Removal of phytic
acid increases the bioavailability of many cations and thus the
nutritional value of the meal [265]. Grinding is the most commonly
used method to remove phytic acid from cereals but has major
drawbacks as it also removes large amounts of minerals and dietary
fibre [265]. In addition, soaking cereals such as pearl millet with
endogenous or exogenous phytase increases the in
vitro solubility of iron and zinc by
2-23% [269]. Removal of the bran during wheat and maize flour milling
or rice milling can significantly increase iron absorption but
results in a significant reduction in the B-group vitamins [270].
Iron absorption from bread made from extra virgin wheat flour is 6
times higher than from whole wheat flour [271].
Many
studies have shown that polyphenolic compounds, mainly found in tea,
coffee, cocoa, and some fruits and vegetables, inhibit iron
absorption [222, 223, 272]. Other dietary inhibitors of iron
absorption include calcium from dairy products and certain proteins
from milk and legumes [273]. Iron absorption is reduced by foods
containing tannins, oxalic and phytic acids, polyphenols, and
compounds with galloyl groups found in tannic and gallic acids in
tea, coffee, and nuts [33, 274, 275]. In addition, manganese, zinc,
lead, chromium, and calcium compete for and alter iron absorption
[276-278]. However, ascorbic acid from fruits and vegetables and
peptides from partially digested muscle tissue from meat, fish, and
poultry can enhance iron absorption and offset some of the negative
effects of phytic acid and polyphenols when consumed in a mixed diet
[225, 279]. Iron
absorption inhibitors shown in Fig.
10.
Therefore,
consumption of a variety of nutrient-rich foods and understanding
factors that enhance or inhibit iron absorption are crucial for
maintenance of optimal iron levels in the blood donor's organism.
This is particularly important for those with increased iron
requirements, i.e. women and adolescents. Balancing enhancers, such
as vitamin C, meat, poultry, and fish, with careful management of
such inhibitors as tannins, calcium, and phytate, will help to
maximise iron absorption and overall health. Further research and
dietary planning are needed to refine these strategies and ensure
effective iron intake from a variety of food sources.
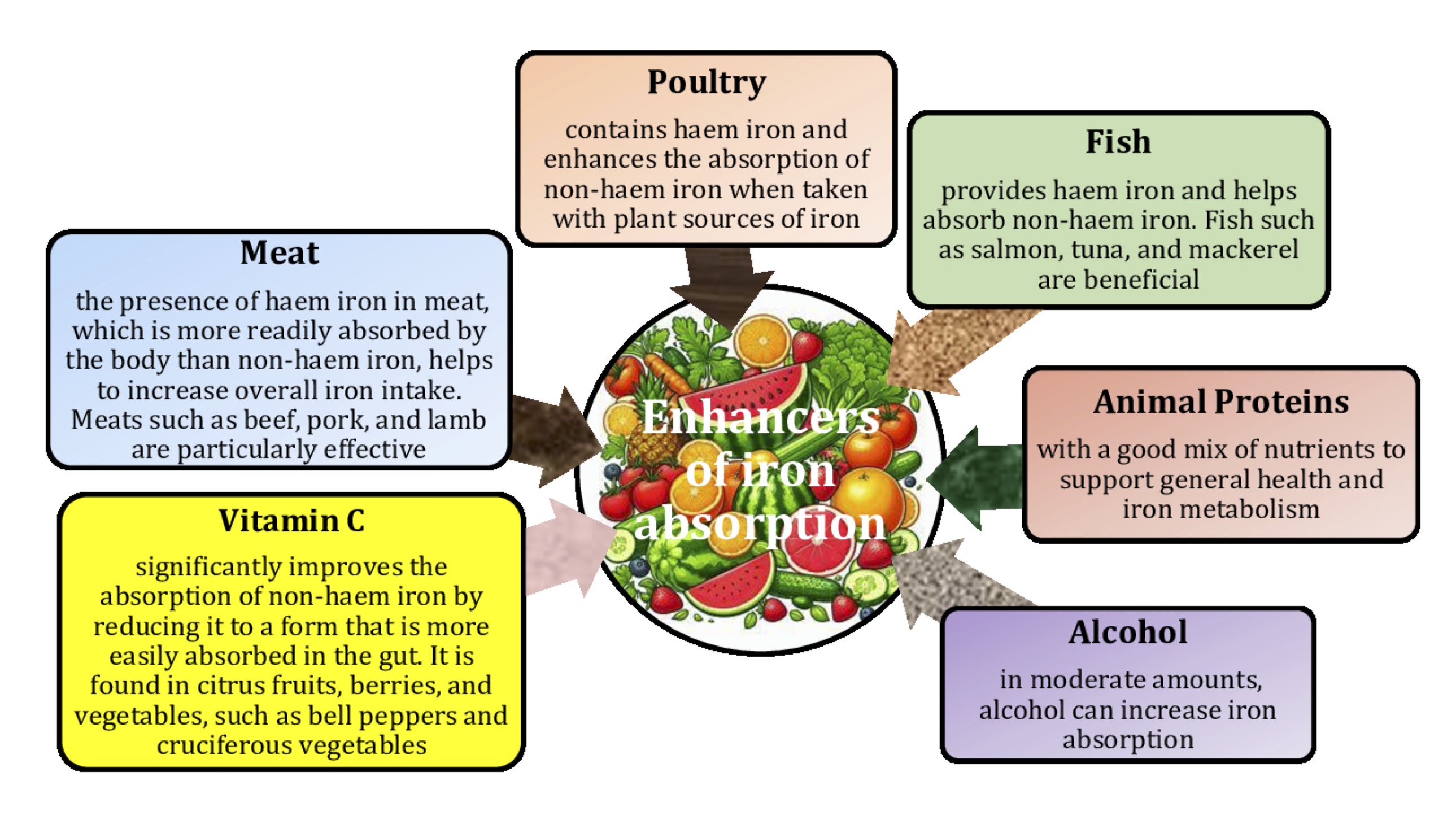
Fig. 9: Enhancers of iron absorption. Understanding factors that influence iron absorption and utilisation is crucial, particularly for those at risk of iron deficiency such as blood donors, pregnant women, and athletes. These factors can be broadly categorised into those that enhance and those that inhibit iron absorption.
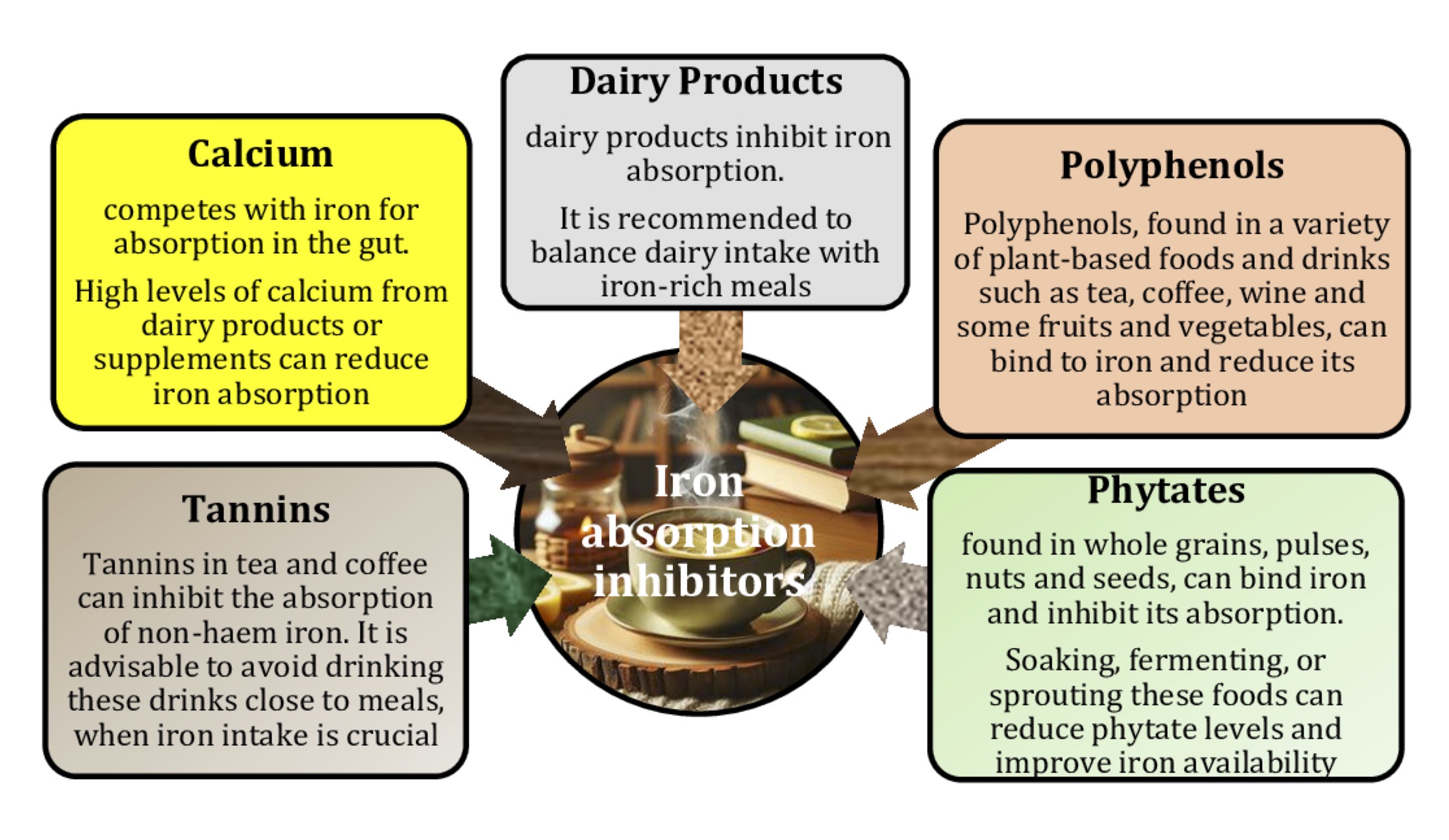
Fig. 10: Iron absorption inhibitors.
Cyancobalamin and folic acid
For
blood donors, ensuring adequate intake of both cyancobalamin (vitamin
B12)
and folic acid (vitamin B11)
is critical for maintenance of an optimal red blood cell count [280,
281]. Erythroblasts require folate and vitamin B12
for proliferation during differentiation. Folate or vitamin B12
deficiency inhibits purine and thymidylate synthesis, impairs DNA
synthesis, and leads to erythroblast apoptosis, resulting in anaemia
due to ineffective erythropoiesis [282]. The classic clinical
manifestation of B12
deficiency is macrocytic or megaloblastic anaemia, characterised by
enlarged erythrocytes and hypersegmented neutrophils. Megaloblastic
anaemia induced by B12
deficiency is essentially identical to megaloblastic anaemia caused
by folic acid deficiency [283].
Vitamin
B12 is
found in foods of animal origin, including fish, meat (e.g. beef,
veal, mutton, and lamb), poultry, eggs, and dairy products (bovine
milk and fermented milk, such as yoghurt and cheese) [284]. The best
dietary sources of vitamin B12
are animal-based foods such as meat, fish, eggs, and dairy products
[285, 286]. Raw liver from beef, pork, and chicken is rich in B12
(52.8, 25.2, and 44.4 μg/100 g wet weight, respectively). The B12
content in raw meat (about 1.0-2.0 μg/100 g wet weight) is higher in
beef than in pork (about 0.5 μg/100 g wet weight) or chicken (<0.5
μg/100 g wet weight) [287]. B12
concentrations in milk from ruminants, such as sheep (0.71 μg/100 g
milk), cow (0.35 μg/100 g milk), and goat (0.06 μg/100 g milk), are
higher than in human milk (0.04 μg/100 g milk) [284]. Raw and cooked
whole hen eggs contain 0.9 μg of B12
per 100 g wet weight of the edible part, with its highest levels in
the yolk [288]. Trace amounts (<0.1 μg/100 g dry weight) of B12
have been found in dried fruiting bodies of black morels, oyster
mushrooms, parasol mushrooms, and porcini mushrooms [289]. Folate is
naturally present in many foods, including vegetables (especially
dark green leafy vegetables), fruits and fruit juices, nuts, beans,
peas, seafood, eggs, dairy products, meat, poultry, and cereals
[290]. Foods with the highest folate content include spinach, liver,
asparagus, and Brussels sprouts (Fig. 11).
Vitamin
B12
plays an important role in the metabolism of methylmalonic acid and
homocysteine, which are essential for DNA synthesis [283, 291].
Cobalamin works with folic acid in the methylation cycle required to
convert homocysteine to methionine [292]. Methionine is an amino acid
used to produce S-adenosylmethionine, a key donor of methyl groups
for various methylation reactions, including those involved in DNA
and RNA synthesis [291]. Folate, in the form of
methylenetetrahydrofolate, is a necessary substrate for the
conversion of uridylate to thymidylate and the subsequent
incorporation of thymidine into DNA. Folate deficiency inhibits DNA
synthesis in blood cell precursors in the bone marrow, preventing
mitosis while allowing cytoplasmic maturation. The result is an
increased cell volume but a reduced number of circulating red blood
cells (i.e. megaloblastic anaemia) [283]. B12
deficiency inhibits the conversion of homocysteine and
methyltetrahydrofolate to methionine and tetrahydrofolate. Folate is
trapped as methyltetrahydrofolate and therefore cannot serve as a
substrate for thymidine synthesis. This results in a functional
folate deficiency and megaloblastic anaemia [283]. In addition to
megaloblastic anaemia, the other classic pathophysiological
manifestation of B12
deficiency is neuronal demyelination, which affects both the
peripheral and central nervous systems [293] and shown in Figs 9 and
10.
Blood
donors on vegetarian or vegan diets may need supplements or fortified
foods to meet their vitamin B12
needs [285]. Meat, fish, eggs, and dairy products as sources of
vitamin B12
and plant foods, such as green leafy vegetables (e.g. spinach and
kale), legumes (e.g. beans and lentils), and fortified cereals, as
sources of folate help to maintain adequate levels of vitamin B12
and folic acid to support effective red blood cell production and
overall health, which is essential for those who donate blood
regularly [284-286, 292].
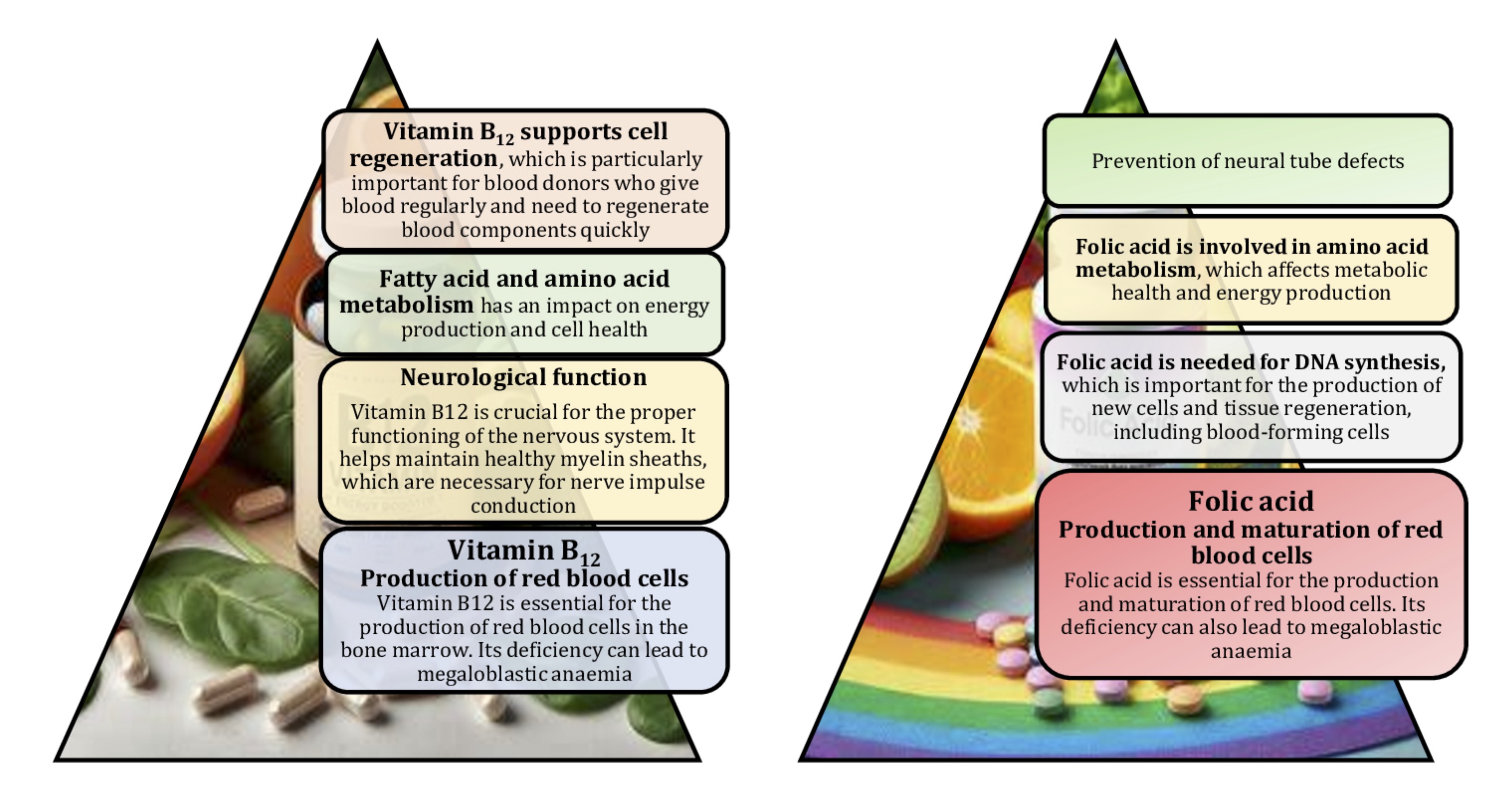
Fig. 11: Vitamin B12 and folic acid are essential for the production of blood components and efficient body function, which is particularly important for blood donors who need optimal regeneration after blood loss.
Vitamins and minerals
Vitamins
A, C, and E, together with essential minerals, such as zinc and
copper, work synergistically to protect cells from oxidative damage,
support immune function, and ensure proper tissue repair [294]. To
maintain donor health and promote overall well-being, it is important
to ensure adequate intake of these nutrients through a balanced diet
or supplements. A diet rich in these vitamins and minerals can help
blood donors to maintain optimal health and support effective
recovery [295, 296].
For
blood donors, vitamins A, C, and E are essential for maintaining
health through their roles as antioxidants and regulators of cellular
processes, reproduction, embryonic development, and growth [160].
Vitamin A (retinol) is essential for maintaining healthy vision,
immune function, and cellular differentiation [297]. At the molecular
level, vitamin A has an impact on gene expression by activating
nuclear receptors, such as retinoic acid receptors, which regulate
the transcription of genes involved in cell growth and immune
responses [298, 299]. This modulation helps to maintain the integrity
of epithelial tissues and supports immune cell function, which is
critical for donors to recover effectively after blood donation
[297]. The recommended dietary allowance (RDA) for men and women is
900 and 700 μg retinol activity equivalents (RAE)/day, respectively.
The tolerable upper intake (UL) for adults is set at 3, 000 μg/day
of preformed vitamin A. The median intake of vitamin A ranges from
744 to 811 μg RAE/day for men and from 530 to 716 μg RAE/day for
women [300]. The main dietary sources of provitamin A are foods of
animal origin, especially liver, meat, milk, dairy products, and
fish. On the other hand, provitamin A carotenoids are found naturally
mainly in yellow, red, and orange fruits and vegetables, such as
carrots, sweet potatoes, squash, and broccoli [301]. The highest
levels of retinol (18000-500 µg/100 g) are found in cod liver oil,
liver, eel, butter, chicken eggs, pecorino cheese, and caviar. The
highest levels of provitamin A carotenoids (36000-3000 µg/100 g) are
found in paprika, parsley, carrots, basil, sweet potatoes, cabbage,
red peppers, yellow squash, mango, and radicchio [248].
Vitamin
C (ascorbic acid) is a potent water-soluble antioxidant that protects
cellular components from free radicals generated during metabolism
and has anti-atherogenic, anti-carcinogenic, and immunomodulatory
effects [302]. In addition, ascorbic acid is a cofactor for several
enzymes, such as dopamine B-monooxygenase or prolyl 4-hydroxylase and
lysyl hydroxylase [303]. It plays a crucial role in the synthesis of
collagen, carnitine, and neurotransmitter biosynthesis [304].
Collagen synthesis is essential for the structural integrity of blood
vessels and skin [305]. Vitamin C works synergistically with vitamin
E to quench free radicals and regenerate reduced vitamin E [160].
Natural sources of vitamin C include sour fruits, green vegetables,
and tomatoes. Good food sources include red and green peppers, kiwi
fruit, broccoli, strawberries, Brussels sprouts, and cantaloupe
[306]. Ascorbic acid can be lost during cooking, as it is a labile
molecule [302]. Vitamin C also enhances the absorption of non-haem
iron from plant foods [99]. This is important for replenishing iron
stores after blood donation.
Vitamin
E (tocopherol) is a fat-soluble antioxidant protecting cell membranes
from oxidative damage by neutralising lipid peroxyl radicals [307].
It also protects polyunsaturated fatty acids in membrane
phospholipids and plasma lipoproteins through its peroxyl radical
scavenging activity [308, 309]. Vitamin E supplements can reduce
measures of lipid peroxidation in subjects with oxidative stress, for
example by reducing urinary F2-isoprostanes in hypercholesterolaemic
subjects [310] and in diabetics [311]. Vitamin E has been shown to
stimulate the body's defences, improve humoral and cellular immune
responses, and enhance phagocytic functions. It has a pronounced
effect in infectious diseases involving immune phagocytosis, but is
less effective in cell-mediated immune defence [307]. Vitamin E is
found in a variety of foods, including nuts, seeds, and vegetable
oils. It is also found in green leafy vegetables and fortified
cereals [307]. Vitamin E works with vitamin C to prevent oxidative
damage and support immune function, helping to stabilise cell
membranes and reduce inflammation [160], which is important for
maintaining the overall health of donors. By reducing oxidative
stress, vitamin E supports the recovery of red blood cells and other
tissues affected by blood donation.
Minerals,
such as zinc and copper, are essential trace elements playing an
important role in maintaining donor health. Zinc is considered
essential for erythropoiesis, along with iron, folate, and vitamin
B12
[312]. It is the second most abundant metal in humans, with a total
of 2-3 g, and is unevenly distributed in different organs and tissues
[313]. Zinc has an impact on many aspects of the immune system, DNA
synthesis, and cell division [314, 315]. It plays a crucial role in
cell proliferation, differentiation, and apoptosis. Intermediate
metabolism, DNA synthesis, reproduction, vision, taste, and cognition
all depend on zinc [316]. Zinc is essential for the normal
development and function of cells that mediate innate immunity,
neutrophils, macrophages, and NK cells. Phagocytosis, intracellular
killing, T and B cell function, and cytokine production are all
affected by zinc deficiency [315]. Its ability to act as an
antioxidant and stabilise membranes suggests a role in preventing
free radical-induced damage during inflammatory processes [316]. Zinc
retards oxidative processes in the long term by inducing the
expression of metallothioneins. These metal-binding cysteine-rich
proteins are responsible for maintaining zinc-related cellular
homeostasis and act as potent electrophilic scavengers and
cytoprotectors [316]. The current recommended dietary allowance (RDA)
for zinc proposed by the Institute of Medicine is 11 mg/day for men
and 8 mg/day for women [251]. Red meat and oysters are rich dietary
sources of zinc [313]. Adequate zinc levels are necessary for proper
immune response and wound healing, which is particularly important
for donors recovering from blood donation.
Copper
is another essential mineral that plays a critical role in
physiological processes, such as respiration, connective tissue
formation, wound repair, macronutrient energy metabolism,
catecholamine biosynthesis, and iron flux [317]. Copper is a
redox-active metal ion serving as a catalytic and structural
co-factor for enzymes involved in energy production, iron uptake,
oxygen transport, cellular metabolism, maturation of peptide
hormones, blood clotting, signal transduction, and a host of other
processes [318]. Copper is also involved in the formation of collagen
and elastin [319], which are important for maintaining healthy blood
vessels and tissues. The Recommended Dietary Allowance (RDA) is 0.9
mg/day for adults. The average daily intake of Cu is estimated to be
between 1.0 and 1.6 mg/day, well above the RDA [320]. The most common
foods containing Cu are shellfish, meat, seeds, nuts, lentils, green
leafy vegetables, and cocoa [321].
Role of a healthy lifestyle in blood donation
The 'healthy donor effect' refers to a bias in health research involving blood donors, where donors tend to be healthier than the general population [322]. This phenomenon occurs because individuals who donate blood are usually required to meet certain health criteria, making them a self-selected group of relatively healthy individuals. As a result, studies of blood donors may not accurately reflect the health status of the wider population, leading to potential methodological problems in research findings. The healthy donor effect, analysed in study [53], is therefore a major methodological challenge in the blood donor health research. By studying its various components, researchers can better understand and manage this bias, achieving more accurate and reliable results [140]. Recognising and correcting for the healthy donor effect is essential to advance the knowledge of the health impact of blood donation and to improve the safety and effectiveness of blood transfusion services [323].
In addition to diet and exercise, proper hydration and sufficient sleep are essential to a healthy lifestyle when donating blood. Hydration is particularly important because blood is mostly water, and adequate fluid intake helps to maintain blood volume and pressure, making the donation process smoother and reducing the risk of dizziness or fainting [324]. Sleep, on the other hand, allows the body to repair and regenerate, which is critical for blood cell recovery and overall energy levels [325, 326]. By prioritising these aspects of a healthy lifestyle, blood donors can ensure that they are in the best possible condition to donate, ultimately contributing to the effectiveness and sustainability of blood donation programmes [327].
Blood donation is known to result in a loss of iron per donation [14] and shown in Figs 2 and 4. Regular physical activity helps to optimise iron absorption and mobilisation, thereby reducing the risk of iron deficiency and associated anaemia, which may affect the donor's ability to continue donating [328, 329] and shown in Fig. 8. Exercise training is known to reduce the circulating ferritin concentration as a biomarker of whole-body iron stores in healthy adults [330, 331]. This is due, at least in part, to increased iron consumption as a result of increased erythropoiesis, which is known to occur with endurance training [329]. Increased erythropoiesis and iron turnover, especially in men [75, 76, 78] compared to women, with regular exercise supports faster recovery of haemoglobin and red blood cell levels after donation. This allows donors to return to their pre-donation health status more quickly, ensuring that they can donate more frequently without adverse effects.
A study by Ziegler et al. [332] showed that individual recovery was variable, but physical performance was recovered 14 days after a standard blood donation, despite the blood haemoglobin concentration remaining lower than at baseline. In another study, blood donation caused a significant decrease in VO2peak for 2-3 weeks. The practical application of this study is that aerobic performance is reduced for up to 3 weeks after blood donation in people of average fitness. However, in an incremental test on a cycle ergometer, there was no effect of blood donation on performance measured by time to fatigue [333]. Therefore, the role of a healthy lifestyle in blood donation is paramount to ensure the well-being of donors and the quality of donated blood.
Regular physical activity and iron metabolism
Physical activity is an important aspect of a healthy lifestyle that benefits blood donation [334]. Regular exercise improves cardiovascular health and circulation and helps to maintain a healthy weight, all of which contribute to a donor's overall fitness [335]. Exercise also stimulates the production of red blood cells, which contributes to faster recovery after donation. However, it is important for donors to balance their physical activity with adequate rest and nutrition, as intense exercise without proper recovery can deplete the body's resources, including iron and other vital nutrients, making recovery after blood donation more difficult [327].
Regular physical activity affects iron metabolism through a number of mechanisms, including enhanced erythropoiesis, increased iron turnover, and improved iron absorption [336]. It is known that regular physical activity stimulates erythropoiesis – the production of red blood cells – to meet the increased oxygen demand of active muscles [337]. This increased erythropoietic activity lowers hepcidin levels, which increases iron absorption and mobilisation from body stores to support red blood cell synthesis [338]. It is important to note that physical activity increases red blood cell turnover, which requires efficient recycling of iron from senescent (old) cells. Macrophages in the spleen and liver break down these cells, releasing iron, which is then transported by transferrin to the bone marrow for the production of new red blood cells [339]. Another mechanism is that regular exercise improves the efficiency of intestinal iron absorption, probably by modulating hepcidin levels and upregulating iron transport proteins, such as DMT1 and ferroportin [340, 341].
In summary, the molecular mechanisms of iron metabolism are closely linked to regular physical activity, which plays a critical role in maintaining iron balance and supporting donor health [342, 343]. Understanding and harnessing this relationship can help to optimise donor health and ensure a consistent and reliable supply of high quality blood to those in need. For example, a nutritious diet rich in essential vitamins and minerals is vital for the production of high-quality red blood cells and effective post-donation recovery [296], and regular physical activity improves iron metabolism and general health, facilitating faster recovery and supporting long-term donor health [344]. In addition, maintenance of psycho-emotional well-being through proper nutrition improves mental health, reduces stress, and enriches the donation experience [86].
Psycho-emotional responses and their relationship with diet
The role of a healthy lifestyle in blood donation is also crucial, and it is important that individuals who donate blood regularly maintain a healthy lifestyle [345], as this has a direct impact on both the quality of the blood donated and the donor's recovery process. The modern diet, often characterised by processed foods and nutrient imbalances, may not meet the specific nutritional needs of blood donors [346]. Therefore, comparing the typical modern diet with the nutritional requirements of blood donors highlights key differences and areas for improvement specific to the donor population. Understanding these differences provides insights into how dietary adjustments can improve the health and performance of blood donors – something that is important not only for donors but also for a wide group of people interested in a healthy lifestyle (Fig. 1).
The process of donating blood can elicit various psycho-emotional responses in donors, ranging from anxiety and stress to feelings of altruism and satisfaction [347]. These responses are controlled by complex molecular mechanisms involving the brain, neurotransmitters, and hormones. In addition, diet can play an important role in modulating these psycho-emotional states, thereby influencing the overall donation experience and the donor recovery process. By supporting mental and emotional well-being throughout the donation process, nutrition plays an important role in modulating these responses. Understanding and harnessing these relationships can help donors to improve their donation experience and recovery, ultimately contributing to the sustainability of blood donation programmes [41, 68, 101].
A healthy lifestyle includes regular and varied physical activity, a balanced diet rich in fresh fruit and vegetables, adequate sleep, and healthy habits, such as avoidance of smoking and excessive alcohol consumption [344, 345]. It also includes mental health through stress management techniques, social interactions, and positive relationships, all of which contribute to the overall well-being and quality of life [348]. Maintaining these elements helps to promote physical fitness, emotional resilience, and a positive outlook on life. A balanced diet rich in essential nutrients, regular physical activity, adequate hydration, and sufficient sleep all contribute to the overall well-being of the donor and the efficiency of the blood donation process. A healthy lifestyle ensures that donors have optimal levels of haemoglobin, iron, and other key components that are essential for the production of high quality blood and the rapid regeneration of blood cells after donation [101].
The main mechanisms of psycho-emotional responses associated with blood donation include the state of mind, hormonal responses, stress-reducing nutrients, hydration, electrolyte balance, and antioxidant-rich foods [349] and demonstrated in Fig. 8. Firstly, serotonin plays a crucial role as a neurotransmitter, often referred to as the 'feel good' neurotransmitter, which regulates mood, reduces anxiety, and promotes emotional well-being [350]. The synthesis of serotonin is dependent on the availability of its precursor, tryptophan, an essential amino acid derived from the diet [351]. Dopamine metabolism is associated with feelings of reward and pleasure. Positive emotions associated with altruistic acts, such as blood donation, can increase dopamine levels [352], thereby increasing the donor's sense of satisfaction and well-being [41]. Some authors highlight the role of cortisol, known as the 'stress hormone', which is released by the adrenal glands in response to stress [353, 354]; elevated cortisol levels can cause anxiety and discomfort. Effective stress management through lifestyle and dietary choices is critical to maintaining balanced cortisol levels [355], especially in blood donors. In recent decades, studies have analysed the multifaceted role of oxytocin, often referred to as the 'love hormone', which is associated with social bonding and positive emotional states [356, 357]. The act of donating blood can stimulate the release of oxytocin, which promotes feelings of connection and altruism [358]. The endocannabinoid system also plays an important role in regulating mood, stress responses, and pain perception [359]. Diets rich in omega-3 fatty acids support the function of the endocannabinoid system [360], potentially helping to modulate stress and anxiety levels in blood donors.
Thus, data from the literature show that fluctuations in serotonin, cortisol and oxytocin levels in blood donors are closely linked to both physiological and psychological responses during and after blood donation [350]. Elevated cortisol levels, particularly in unhealthy donors, indicate increased stress and anxiety, reflecting the body's response to the perceived challenge of donating [353, 354]. Conversely, decreased serotonin levels during the process indicate mood instability and stress, contributing to discomfort. On the other hand, the altruistic nature of blood donation, which promotes social bonding and stress reduction, has been shown to increase oxytocin levels in healthy donors, indicating a positive emotional response [356, 357]. These findings from the literature show how these psychosocial parameters interact and contribute to the variability in donor response.
Some studies have focused on the link between diet and nutritional support for good mood, particularly through tryptophan-rich foods that support serotonin production [361, 362]. Such foods as turkey, eggs, nuts, and seeds are high in tryptophan; it is important for maintaining adequate levels of serotonin, which can help to reduce anxiety and improve mood, thereby enhancing the blood donation experience [351]. Another important amino acid is tyrosine, which is found in chicken, cheese, and soy products. Tyrosine acts as a precursor of dopamine and contributes to feelings of reward and satisfaction after donation when included in a diet rich in tyrosine-rich foods [350]. The stress-reducing nutrients include omega-3 fatty acids found in fish, flaxseed, and walnuts, which reduce inflammation and cortisol levels, improving emotional resilience in donors [363]. In addition, magnesium, found in leafy greens, nuts, and whole grains, supports the regulation of the HPA axis, which is critical for managing cortisol release and mitigating stress responses [364].
Thus, understanding the impact of diet on psycho-emotional health underscores the importance of comprehensive dietary strategies tailored to blood donors. By promoting a nutrient-rich diet, donors can better cope with the emotional and psychological demands of regular blood donation. This approach not only supports their immediate recovery, but also contributes to their long-term mental and emotional well-being. Recognising and addressing the psycho-emotional needs of donors through targeted nutritional interventions can improve the overall donation experience and ensure that donors remain healthy, motivated, and able to consistently provide high quality blood donations.
Practical implications for blood donors emphasise the importance of maintaining a nutrient-rich diet tailored to individual needs, particularly the risk of iron deficiency, which is common among frequent donors. Donors should prioritise iron-rich foods such as lean meats, green leafy vegetables and fortified cereals, complemented by vitamin C sources to enhance iron absorption. Gender-specific dietary recommendations are also critical, as women are more susceptible to iron depletion due to menstruation, requiring additional attention to iron intake. Donors should be educated about the importance of hydration, adequate protein intake, and supplementation with essential vitamins and minerals before and after donation to support recovery and overall well-being. These guidelines aim to promote safe and sustainable donation practices while ensuring the health of the donor.
Conclusion
Based on a systematic search of PubMed, Web of Science, Scopus, Google Scholar, Cochrane Library and ScienceDirect databases, the article provides a comprehensive review of how diet affects the health and performance of blood donors, explains the underlying molecular mechanisms, and offers practical recommendations to support donor well-being and the effectiveness of blood donation programmes worldwide. This article highlights the fact that the diet of blood donors is influenced by gender, as men and women have different nutritional needs and physiological responses, particularly with regard to iron levels and recovery. This highlights the importance of taking gender differences into account when developing dietary recommendations to support the well-being of blood donors. The methodology and approach of this review provides valuable evidence for understanding the complex relationship between health, nutrition, and blood quality and highlights the importance of comprehensive care to ensure that donors remain healthy and able to provide high quality blood donations.
This review provides an analysis of the current literature on improving the health and nutrition of blood donors, examining key aspects that affect the quality of donated blood and donor well-being. Understanding these differences is critical for tailoring dietary recommendations and supplements to maintain optimal iron levels in all donors. Effective iron absorption facilitated by key nutrients, such as vitamin C, plays an important role in replenishing iron stores after donation, ensuring faster recovery and long-term donor health. The review presents evidence on the importance of a balanced diet rich in essential nutrients, minerals such as iron, copper, and zinc, as well as vitamin B12 and folic acid, which are essential for blood donors and crucial for red blood cell production and overall blood quality.
The effects of regular physical activity on iron metabolism and general health were also discussed, highlighting how it improves recovery after donation and contributes to donor well-being. Regular blood donation can stress the immune system; therefore, it is important to monitor and maintain donors' long-term health through adequate intake of iron and other key nutrients that support immune function. Psycho-emotional well-being is also linked to diet, with good dietary habits improving mental health, reducing stress, and improving the overall donation experience. To maintain optimal health in blood donors, it is important to include a variety of nutrient-rich foods and to understand factors that promote or inhibit iron absorption.
The healthy donor effect, i.e. a bias in health research where blood donors tend to be healthier than the general population, is discussed in this review. This effect highlights the need for tailored health and nutrition strategies to maintain this higher standard of health among donors. This review assesses whether food fortification with iron could be a cost-effective solution to reduce iron deficiency in blood transfusion recipients and ensure sufficient iron levels to maintain their health and blood quality.
Acknowledgements
Author Contributions
N.K.,
H.T., M.G. conceived the concept of the review; N.K., H.T. developed
the search strategy; N.K., H.T. coordinated data selection,
extraction, analysis, and interpretation; N.K., H.T. critically
reviewed the manuscript; N.K., H.T. drafted the final manuscript.
Funding
This research received no specific grant from any funding agency,
commercial or not-for-profit sectors.
Disclosure Statement
The authors have no conflicts of interest to declare.
Disclosure of AI Assistance
No AI tools or AI-generated content were used in the creation of this
article text. The content was entirely developed, written, and edited
by the authors.
References
| 1 | Hughes SD, France CL, West-Mitchell KA, Pina T, McElfresh D, Sayers M, Bryant BJ; NHLBI SoS Working Group #1. Blood Donors and the Supply. Advancing Understandings of Blood Donation Motivation and Behavior. Transfus Med Rev. 2023;37:150780 https://doi.org/10.1016/j.tmrv.2023.150780.
https://doi.org/10.1016/j.tmrv.2023.150780 |
| 2 | Studte S, Clement M, Soliman M, Boenigk S. Blood donors and their changing engagement in other prosocial behaviors. Transfusion. 2019;59(3):1002-1015 https://doi.org/10.1111/trf.15085.
https://doi.org/10.1111/trf.15085 |
| 3 | Chen J, Ou-Yang J, Xie G, Liang H, Fan Y, Gao R, Li S, Rong X, He B, Bei C, Fu Y. Problems and challenges: development of blood transfusion services in Mainland China within the context of health-care system reform. Transfus Med. 2019;29:253-261 https://doi.org/10.1111/tme.12618.
https://doi.org/10.1111/tme.12618 |
| 4 | Bednall TC, Bove LL, Cheetham A, Murray AL. A systematic review and meta-analysis of antecedents of blood donation behavior and intentions. Soc Sci Med. 2013;96:86-94 https://doi.org/10.1016/j.socscimed.2013.07.022.
https://doi.org/10.1016/j.socscimed.2013.07.022 |
| 5 | Fujimoto A, Suzuki R, Orihara K, Iida M, Yamashita T, Nagafuji K, Kanamori H, Kodera Y, Miyamura K, Okamoto S, Hino M. Health-related quality of life in peripheral blood stem cell donors and bone marrow donors: a prospective study in Japan. Int J Hematol. 2020;111:840-850 https://doi.org/10.1007/s12185-020-02852-7.
https://doi.org/10.1007/s12185-020-02852-7 |
| 6 | To L, Dunnington T, Thomas C, Love K, McCullough J, Riley W. The United States' potential blood donor pool: updating the prevalence of donor-exclusion factors on the pool of potential donors. Transfusion. 2020;60(1):206-215 https://doi.org/10.1111/trf.15573.
https://doi.org/10.1111/trf.15573 |
| 7 | Peña-Carillo HI, Rosa-Zamboni D, López-Victoria AB, López-Martínez B, Guerrero-Díaz AC. Strategies to recover blood donors during the COVID-19 pandemic: a tertiary level hospital experience. Estrategias para recuperar donantes de sangre durante la pandemia por COVID-19: experiencia de un hospital de tercer nivel. Bol Med Hosp Infant Mex. 2022;79:300-309 https://doi.org/10.24875/BMHIM.21000237.
https://doi.org/10.24875/BMHIM.21000237 |
| 8 | Guarnaccia C, Giannone F, Falgares G, Caligaris AO, Sales-Wuillemin E. Differences in social representation of blood donation between donors and non-donors: an empirical study. Blood Transfus. 2016;14:487-489 https://doi.org/10.2450/2015.0048-15.
|
| 9 | Paalvast Y, Moazzen S, Sweegers M, Hogema B, Janssen M, van den Hurk K. A computational model for prediction of ferritin and haemoglobin levels in blood donors. Br J Haematol. 2022;199(1):143-152 https://doi.org/10.1111/bjh.18367.
https://doi.org/10.1111/bjh.18367 |
| 10 | Meulenbeld A, Ramondt S, Sweegers MG, Quee FA, Prinsze FJ, Hoogendijk EO, Swinkels DW, van den Hurk K. Effectiveness of ferritin-guided donation intervals in whole-blood donors in the Netherlands (FIND'EM): a stepped-wedge cluster-randomised trial. Lancet. 2024;404(10447):31-43 https://doi.org/10.1016/S0140-6736(24)01085-7.
https://doi.org/10.1016/S0140-6736(24)01085-7 |
| 11 | Mahida VI, Bhatti A, Gupte SC. Iron status of regular voluntary blood donors. Asian J Transfus Sci. 2008;2(1):9-12 https://doi.org/10.4103/0973-6247.39504.
https://doi.org/10.4103/0973-6247.39504 |
| 12 | Karacan E, Cengiz Seval G, Aktan Z, Ayli M, Palabiyikoglu R. Blood donors and factors impacting the blood donation decision: motives for donating blood in Turkish sample. Transfus Apher Sci. 2013;49(3):468-473 https://doi.org/10.1016/j.transci.2013.04.044.
https://doi.org/10.1016/j.transci.2013.04.044 |
| 13 | Spencer BR, Mast AE. Iron status of blood donors. Curr Opin Hematol. 2022;29:310-316 https://doi.org/10.1097/MOH.0000000000000733.
https://doi.org/10.1097/MOH.0000000000000733 |
| 14 | Mantadakis E, Panagopoulou P, Kontekaki E, Bezirgiannidou Z, Martinis G. Iron Deficiency and Blood Donation: Links, Risks and Management. J Blood Med. 2022;13:775-786 https://doi.org/10.2147/JBM.S375945.
https://doi.org/10.2147/JBM.S375945 |
| 15 | Pittori C, Buser A, Gasser UE, Sigle J, Job S, Rüesch M, Tichelli A, Infanti L. A pilot iron substitution programme in female blood donors with iron deficiency without anaemia. Vox Sang. 2011;100(3):303-311 https://doi.org/10.1111/j.1423-0410.2010.01427.x.
https://doi.org/10.1111/j.1423-0410.2010.01427.x |
| 16 | Cable RG, Glynn SA, Kiss JE, Mast AE, Steele WR, Murphy EL, Wright DJ, Sacher RA, Gottschall JL, Vij V, Simon TL, NHLBI Retrovirus Epidemiology Donor Study-II. Iron deficiency in blood donors: analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51:511-522 https://doi.org/10.1111/j.1537-2995.2010.02865.x.
https://doi.org/10.1111/j.1537-2995.2010.02865.x |
| 17 | Kiss JE, Vassallo RR. How do we manage iron deficiency after blood donation?. Br J Haematol. 2018;181:590-603 https://doi.org/10.1111/bjh.15136.
https://doi.org/10.1111/bjh.15136 |
| 18 | Vuk T, Garraud O, Politis C. Post-donation information management. Transfus Clin Biol. 2021;28:407-413 https://doi.org/10.1016/j.tracli.2021.08.006.
https://doi.org/10.1016/j.tracli.2021.08.006 |
| 19 | Vuk T, Barišić M, Očić T, Hećimović A, Sarlija D, Jukić I. Could the frequency of lipaemic donations be reduced by educational activities?. Blood Transfus. 2012;10:555-556 https://doi.org/10.2450/2012.0138-11.
|
| 20 | Ettinger S. Diet, Gut Microbiome, and Cognitive Decline. Curr Nutr Rep. 2022;11:643-652 https://doi.org/10.1007/s13668-022-00435-y.
https://doi.org/10.1007/s13668-022-00435-y |
| 21 | Farley MA, Smith PD, Mahoney AW, West DW, Post JR. Adult dietary characteristics affecting iron intake: a comparison based on iron density. J Am Diet Assoc. 1987;87:184-189.
https://doi.org/10.1016/S0002-8223(21)03088-1 |
| 22 | Institute of Medicine (US) Panel on Micronutrients. 2001 Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US).
|
| 23 | Swanson CA. Iron intake and regulation: implications for iron deficiency and iron overload. Alcohol. 2003;30:99-102 https://doi.org/10.1016/s0741-8329(03)00103-4.
https://doi.org/10.1016/S0741-8329(03)00103-4 |
| 24 | Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3:12-33 https://doi.org/10.3390/pharmaceutics3010012.
https://doi.org/10.3390/pharmaceutics3010012 |
| 25 | Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part 1: molecular basis of iron homoeostasis. J Clin Pathol. 2011;64:281-286 https://doi.org/10.1136/jcp.2010.079046.
https://doi.org/10.1136/jcp.2010.079046 |
| 26 | Zeidan RS, Han SM, Leeuwenburgh C, Xiao R. Iron homeostasis and organismal aging. Ageing Res Rev. 2021;72:101510 https://doi.org/10.1016/j.arr.2021.101510.
https://doi.org/10.1016/j.arr.2021.101510 |
| 27 | Waldvogel-Abramowski S, Waeber G, Gassner C. Physiology of iron metabolism. Transfus Med Hemother. 2014;41:213-221 https://doi.org/10.1159/000362888.
https://doi.org/10.1159/000362888 |
| 28 | Syed BA, Sargent PJ, Farnaud S, Evans RW. An overview of molecular aspects of iron metabolism. Hemoglobin. 2006;30:69-80 https://doi.org/10.1080/03630260500455318.
https://doi.org/10.1080/03630260500455318 |
| 29 | Piskin E, Cianciosi D, Gulec S, Tomas M, Capanoglu E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega. 2022;7:20441-20456 https://doi.org/10.1021/acsomega.2c01833.
https://doi.org/10.1021/acsomega.2c01833 |
| 30 | Hurrell RF. Preventing iron deficiency through food fortification. Nutr Rev. 1997;55:210-222 https://doi.org/10.1111/j.1753-4887.1997.tb01608.x.
https://doi.org/10.1111/j.1753-4887.1997.tb01608.x |
| 31 | Roughead ZK, Zito CA, Hunt JR. Initial uptake and absorption of nonheme iron and absorption of heme iron in humans are unaffected by the addition of calcium as cheese to a meal with high iron bioavailability. Am J Clin Nutr. 2002;76:419-425 https://doi.org/10.1093/ajcn/76.2.419.
https://doi.org/10.1093/ajcn/76.2.419 |
| 32 | Young I, Parker HM, Rangan A, Prvan T, Cook RL, Donges CE, Steinbeck KS, O'Dwyer NJ, Cheng HL, Franklin JL, O'Connor HT. Association between Haem and Non-Haem Iron Intake and Serum Ferritin in Healthy Young Women. Nutrients. 2018;10:81 https://doi.org/10.3390/nu10010081.
https://doi.org/10.3390/nu10010081 |
| 33 | Zijp IM, Korver O, Tijburg LB. Effect of tea and other dietary factors on iron absorption. Crit Rev Food Sci Nutr. 2000;40:371-398 https://doi.org/10.1080/10408690091189194.
https://doi.org/10.1080/10408690091189194 |
| 34 | Cepeda-Lopez AC, Melse-Boonstra A, Zimmermann MB, Herter-Aeberli I. In overweight and obese women, dietary iron absorption is reduced and the enhancement of iron absorption by ascorbic acid is one-half that in normal-weight women. Am J Clin Nutr. 2015;102:1389-1397 https://doi.org/10.3945/ajcn.114.099218.
https://doi.org/10.3945/ajcn.114.099218 |
| 35 | Lönnerdal B. Calcium and iron absorption - mechanisms and public health relevance. Int J Vitam Nutr Res. 2010;80:293-299 https://doi.org/10.1024/0300-9831/a000036.
https://doi.org/10.1024/0300-9831/a000036 |
| 36 | Jaramillo Á, Briones L, Andrews M, Arredondo M, Olivares M, Brito A, Pizarro F. Effect of phytic acid, tannic acid and pectin on fasting iron bioavailability both in the presence and absence of calcium. J Trace Elem Med Biol. 2015;30:112-117 https://doi.org/10.1016/j.jtemb.2014.11.005.
https://doi.org/10.1016/j.jtemb.2014.11.005 |
| 37 | Reeves AJ, McEvoy MA, MacDonald-Wicks LK, Barker D, Attia J, Hodge AM, Patterson AJ. Calculation of Haem Iron Intake and Its Role in the Development of Iron Deficiency in Young Women from the Australian Longitudinal Study on Women's Health. Nutrients. 2017;9:515 https://doi.org/10.3390/nu9050515.
https://doi.org/10.3390/nu9050515 |
| 38 | Hallberg L. Iron requirements and bioavailability of dietary iron. Experientia Suppl. 1983;44:223-244 https://doi.org/10.1007/978-3-0348-6540-1_13.
https://doi.org/10.1007/978-3-0348-6540-1_13 |
| 39 | Yiannikourides A, Latunde-Dada GO. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines (Basel). 2019;6:85 https://doi.org/10.3390/medicines6030085.
https://doi.org/10.3390/medicines6030085 |
| 40 | Montoro-Huguet MA, Santolaria-Piedrafita S, Cañamares-Orbis P, García-Erce JA. Iron Deficiency in Celiac Disease: Prevalence, Health Impact, and Clinical Management. Nutrients. 2021;13:3437 https://doi.org/10.3390/nu13103437.
https://doi.org/10.3390/nu13103437 |
| 41 | Chen WL. Body Donation Registration in Taiwan: Reasons and Associated Psychological Factors. Healthcare (Basel). 2023;11:969 https://doi.org/10.3390/healthcare11070969.
https://doi.org/10.3390/healthcare11070969 |
| 42 | Feng W, Yun W, Le W, Zhi-Guo X, Hai-Ying Y, Shu-Fang W, Zhen-Yan W, Yi-Zhu C, Quan S, Jing-Xian F. The influence of demographic and lifestyle factors on blood donation delay among student population: a retrospective study. Front Public Health. 2023;11:1297472 https://doi.org/10.3389/fpubh.2023.1297472.
https://doi.org/10.3389/fpubh.2023.1297472 |
| 43 | Blood Donor Selection: Guidelines on Assessing Donor Suitability for Blood Donation. Geneva: World Health Organization; 2012 4, General donor assessment. Available from: https://www.ncbi.nlm.nih.gov/books/NBK138219/.
|
| 44 | Hadjesfandiari N, Khorshidfar M, Devine DV. Current Understanding of the Relationship between Blood Donor Variability and Blood Component Quality. Int J Mol Sci. 2021;22:3943 https://doi.org/10.3390/ijms22083943.
https://doi.org/10.3390/ijms22083943 |
| 45 | Trouern-Trend JJ, Cable RG, Badon SJ, Newman BH, Popovsky MA. A case-controlled multicenter study of vasovagal reactions in blood donors: influence of sex, age, donation status, weight, blood pressure, and pulse. Transfusion. 1999;39:316-320 https://doi.org/10.1046/j.1537-2995.1999.39399219291.x.
https://doi.org/10.1046/j.1537-2995.1999.39399219291.x |
| 46 | Newman BH. Donor reactions and injuries from whole blood donation. Transfus Med Rev. 1997;11(1):64-75 https://doi.org/10.1016/s0887-7963(97)80011-9.
https://doi.org/10.1016/S0887-7963(97)80011-9 |
| 47 | Zervou EK, Ziciadis K, Karabini F, Xanthi E, Chrisostomou E, Tzolou A. Vasovagal reactions in blood donors during or immediately after blood donation. Transfus Med. 2005;15:389-394 https://doi.org/10.1111/j.1365-3148.2005.00600.x.
https://doi.org/10.1111/j.1365-3148.2005.00600.x |
| 48 | Philip J, Sarkar RS, Jain N. A single-centre study of vasovagal reaction in blood donors: Influence of age, sex, donation status, weight, total blood volume and volume of blood collected. Asian J Transfus Sci. 2014;8:43-46 https://doi.org/10.4103/0973-6247.126690.
https://doi.org/10.4103/0973-6247.126690 |
| 49 | Yadav K, Singh A, Jaryal AK, Coshic P, Chatterjee K, Deepak KK. Modulation of cardiac autonomic tone in non-hypotensive hypovolemia during blood donation. J Clin Monit Comput. 2017;31:739-746 https://doi.org/10.1007/s10877-016-9912-y.
https://doi.org/10.1007/s10877-016-9912-y |
| 50 | Tondon R, Pandey P, Chaudhary R. Vasovagal reactions in 'at risk' donors: a univariate analysis of effect of age and weight on the grade of donor reactions. Transfus Apher Sci. 2008;39:95-99 https://doi.org/10.1016/j.transci.2008.07.010.
https://doi.org/10.1016/j.transci.2008.07.010 |
| 51 | Kiss JE: Laboratory and genetic assessment of iron deficiency in blood donors. Clin Lab Med. 2015;35:73-91 https://doi.org/10.1016/j.cll.2014.10.011.
https://doi.org/10.1016/j.cll.2014.10.011 |
| 52 | Chueca M, Bouvet G, Duron-Martinaud S, Doyen M, Poirrier L, Martinaud C. Iron-deficiency among blood donors: Donors' opinion on iron supplementation strategy. Transfus Clin Biol. 2020;27:218-221 https://doi.org/10.1016/j.tracli.2020.08.004.
https://doi.org/10.1016/j.tracli.2020.08.004 |
| 53 | Brodersen T, Rostgaard K, Lau CJ, Juel K, Erikstrup C, Nielsen KR, Ostrowski SR, Titlestad K, Saekmose SG, Pedersen OBV, Hjalgrim H. The healthy donor effect and survey participation, becoming a donor and donor career. Transfusion. 2023;63:143-155 https://doi.org/10.1111/trf.17190.
https://doi.org/10.1111/trf.17190 |
| 54 | Kiss JE, Birch RJ, Steele WR, Wright DJ, Cable RG. Quantification of body iron and iron absorption in the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2017;57:1656-1664 https://doi.org/10.1111/trf.14133.
https://doi.org/10.1111/trf.14133 |
| 55 | Garcia-Casal MN, Pasricha SR, Martinez RX, Lopez-Perez L, Peña-Rosas JP. Serum or plasma ferritin concentration as an index of iron deficiency and overload. Cochrane Database Syst Rev. 2021;5:CD011817 https://doi.org/10.1002/14651858.CD011817.pub2.
https://doi.org/10.1002/14651858.CD011817.pub2 |
| 56 | Sukhbaatar N, Weichhart T. Iron Regulation: Macrophages in Control. Pharmaceuticals (Basel). 2018;11:137 https://doi.org/10.3390/ph11040137.
https://doi.org/10.3390/ph11040137 |
| 57 | Lobo AR, Cocato ML, De Sá LR, Colli C. Dietary iron overload: short- and long-term effects on cecal morphometry in growing rats. J Nutr Sci Vitaminol (Tokyo). 2014;60:397-402 https://doi.org/10.3177/jnsv.60.397.
https://doi.org/10.3177/jnsv.60.397 |
| 58 | Kim CH, Leitch HA. Iron overload-induced oxidative stress in myelodysplastic syndromes and its cellular sequelae. Crit Rev Oncol Hematol. 2021;163:103367 https://doi.org/10.1016/j.critrevonc.2021.103367.
https://doi.org/10.1016/j.critrevonc.2021.103367 |
| 59 | Karim A, Bajbouj K, Shafarin J, Qaisar R, Hall AC, Hamad M. Iron Overload Induces Oxidative Stress, Cell Cycle Arrest and Apoptosis in Chondrocytes. Front Cell Dev Biol. 2022;10:821014 https://doi.org/10.3389/fcell.2022.821014.
https://doi.org/10.3389/fcell.2022.821014 |
| 60 | Kawabata T. Iron-Induced Oxidative Stress in Human Diseases. Cells. 2022;11:2152 https://doi.org/10.3390/cells11142152.
https://doi.org/10.3390/cells11142152 |
| 61 | Ito M, Sawada H, Ohishi K, Yoshida Y, Yokoi W, Watanabe T, Yokokura T. Suppressive effects of bifidobacteria on lipid peroxidation in the colonic mucosa of iron-overloaded mice. J Dairy Sci. 2001;84:1583-1589 https://doi.org/10.3168/jds.S0022-0302(01)74591-2.
https://doi.org/10.3168/jds.S0022-0302(01)74591-2 |
| 62 | Ito M, Ohishi K, Yoshida Y, Yokoi W, Sawada H. Antioxidative effects of lactic acid bacteria on the colonic mucosa of iron-overloaded mice. J Agric Food Chem. 2003;51:4456-4460 https://doi.org/10.1021/jf0261957.
https://doi.org/10.1021/jf0261957 |
| 63 | Clamp JR, Creeth JM. Some non-mucin components of mucus and their possible biological roles. Ciba Found Symp. 1984;109:121-136 https://doi.org/10.1002/9780470720905.ch9.
https://doi.org/10.1002/9780470720905.ch9 |
| 64 | Gao G, Li J, Zhang Y, Chang YZ. Cellular Iron Metabolism and Regulation. Adv Exp Med Biol. 2019;1173:21-32 https://doi.org/10.1007/978-981-13-9589-5_2.
https://doi.org/10.1007/978-981-13-9589-5_2 |
| 65 | Xing G, Meng L, Cao S. PPARα alleviates iron overload-induced ferroptosis in mouse liver. EMBO Rep. 2022;23(8):e52280 https://doi.org/10.15252/embr.202052280.
https://doi.org/10.15252/embr.202052280 |
| 66 | Stangerup I, Kramp NL, Ziegler AK, Dela F, Magnussen K, Helge JW. Temporary impact of blood donation on physical performance and hematologic variables in women. Transfusion. 2017;57(8):1905-1911 https://doi.org/10.1111/trf.14121.
https://doi.org/10.1111/trf.14121 |
| 67 | Schreiber GB, Brinser R, Rosa-Bray M, Yu ZF, Simon T. Frequent source plasma donors are not at risk of iron depletion: the Ferritin Levels in Plasma Donor (FLIPD) study. Transfusion. 2018;58:951-959 https://doi.org/10.1111/trf.14489.
https://doi.org/10.1111/trf.14489 |
| 68 | Prados Madrona D, Fernández Herrera MD, Prados Jiménez D, Gómez Giraldo S, Robles Campos R. Women as whole blood donors: offers, donations and deferrals in the province of Huelva, south-western Spain. Blood Transfus. 2014;12 Suppl 1(Suppl 1):s11-s20 https://doi.org/10.2450/2012.0117-12.
|
| 69 | Mast AE. Low hemoglobin deferral in blood donors. Transfus Med Rev. 2014;28:18-22 https://doi.org/10.1016/j.tmrv.2013.11.001.
https://doi.org/10.1016/j.tmrv.2013.11.001 |
| 70 | Badenhorst CE, Forsyth AK, Govus AD. A contemporary understanding of iron metabolism in active premenopausal females. Front Sports Act Living. 2022;4:903937 https://doi.org/10.3389/fspor.2022.903937.
https://doi.org/10.3389/fspor.2022.903937 |
| 71 | Lehtihet M, Bonde Y, Beckman L, Berinder K, Hoybye C, Rudling M, Sloan JH, Konrad RJ, Angelin B. Circulating Hepcidin-25 Is Reduced by Endogenous Estrogen in Humans. PLoS One 2016;11:e0148802 https://doi.org/10.1371/journal.pone.0148802.
https://doi.org/10.1371/journal.pone.0148802 |
| 72 | Ćurko-Cofek B, Grubić Kezele T, Barac-Latas V. Hepcidin and metallothioneins as molecular base for sex-dependent differences in clinical course of experimental autoimmune encephalomyelitis in chronic iron overload. Med Hypotheses. 2017;107:51-54 https://doi.org/10.1016/j.mehy.2017.07.022.
https://doi.org/10.1016/j.mehy.2017.07.022 |
| 73 | Bajbouj K, Shafarin J, Allam H, Madkour M, Awadallah S, El-Serafy A, Sandeep D, Hamad M. Elevated Levels of Estrogen Suppress Hepcidin Synthesis and Enhance Serum Iron Availability in Premenopausal Women. Exp Clin Endocrinol Diabetes. 2018;126(7):453-459 https://doi.org/10.1055/s-0043-124077.
https://doi.org/10.1055/s-0043-124077 |
| 74 | Hamad M, Bajbouj K, Taneera J. The Case for an Estrogen-iron Axis in Health and Disease. Exp Clin Endocrinol Diabetes. 2020;128(4):270-277 https://doi.org/10.1055/a-0885-1677.
https://doi.org/10.1055/a-0885-1677 |
| 75 | Mancera-Soto E, Ramos-Caballero DM, Magalhaes J, Chaves Gomez S, Schmidt WFJ, Cristancho-Mejía E. Quantification of testosterone-dependent erythropoiesis during male puberty. Exp Physiol. 2021;106(7):1470-1481 https://doi.org/10.1113/EP089433.
https://doi.org/10.1113/EP089433 |
| 76 | Mancera-Soto EM, Ramos-Caballero DM, Rojas J JA, Duque L, Chaves-Gomez S, Cristancho-Mejía E, Schmidt WF. Hemoglobin Mass, Blood Volume and VO2max of Trained and Untrained Children and Adolescents Living at Different Altitudes. Front Physiol. 2022;13:892247 https://doi.org/10.3389/fphys.2022.892247.
https://doi.org/10.3389/fphys.2022.892247 |
| 77 | Beggs LA, Yarrow JF, Conover CF, Meuleman JR, Beck DT, Morrow M, Zou B, Shuster JJ, Borst SE. Testosterone alters iron metabolism and stimulates red blood cell production independently of dihydrotestosterone. Am J Physiol Endocrinol Metab. 2014;307(5):E456-E461 https://doi.org/10.1152/ajpendo.00184.2014.
https://doi.org/10.1152/ajpendo.00184.2014 |
| 78 | Waalen J, von Löhneysen K, Lee P, Xu X, Friedman JS. Erythropoietin, GDF15, IL6, hepcidin and testosterone levels in a large cohort of elderly individuals with anaemia of known and unknown cause. Eur J Haematol. 2011;87(2):107-116 https://doi.org/10.1111/j.1600-0609.2011.01631.x.
https://doi.org/10.1111/j.1600-0609.2011.01631.x |
| 79 | Saiz MP, Marti MT, Mitjavila MT, Planas J. Intestinal iron absorption in chickens : II. Effect of sex. Biol Trace Elem Res. 1980;2(4):269-280 https://doi.org/10.1007/BF02783825.
https://doi.org/10.1007/BF02783825 |
| 80 | Rushton DH, Barth JH. What is the evidence for gender differences in ferritin and haemoglobin?. Crit Rev Oncol Hematol. 2010;73(1):1-9 https://doi.org/10.1016/j.critrevonc.2009.03.010.
https://doi.org/10.1016/j.critrevonc.2009.03.010 |
| 81 | Bachman E, Feng R, Travison T, Li M, Olbina G, Ostland V, Ulloor J, Zhang A, Basaria S, Ganz T, Westerman M, Bhasin S. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. 2010;95(10):4743-4747 https://doi.org/10.1210/jc.2010-0864.
https://doi.org/10.1210/jc.2010-0864 |
| 82 | Ikeda Y, Tajima S, Izawa-Ishizawa Y, Kihira Y, Ishizawa K, Tomita S, Tsuchiya K, Tamaki T. Estrogen regulates hepcidin expression via GPR30-BMP6-dependent signaling in hepatocytes. PLoS One. 2012;7(7):e40465 https://doi.org/10.1371/journal.pone.0040465.
https://doi.org/10.1371/journal.pone.0040465 |
| 83 | Lee BK, Kim Y. Sex-specific Profiles of Blood Metal Levels Associated with Metal-Iron Interactions. Saf Health Work. 2014;5(3):113-117 https://doi.org/10.1016/j.shaw.2014.06.005.
https://doi.org/10.1016/j.shaw.2014.06.005 |
| 84 | Sayers M. Donor iron nutrition: Which precaution prevails?. Transfusion. 2021;61(1):313-317 https://doi.org/10.1111/trf.16158.
https://doi.org/10.1111/trf.16158 |
| 85 | Popkin BM, D'Anci KE, Rosenberg IH. Water, hydration, and health. Nutr Rev. 2010;68(8):439-458 https://doi.org/10.1111/j.1753-4887.2010.00304.x.
https://doi.org/10.1111/j.1753-4887.2010.00304.x |
| 86 | Firth J, Gangwisch JE, Borisini A, Wootton RE, Mayer EA. Food and mood: how do diet and nutrition affect mental wellbeing? BMJ. 2020;369:m2382 https://doi.org/10.1136/bmj.m2382.
https://doi.org/10.1136/bmj.m2382 |
| 87 | Cena H, Calder PC. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients. 2020;12(2):334 https://doi.org/10.3390/nu12020334.
https://doi.org/10.3390/nu12020334 |
| 88 | Sasidharan Nair V, Huehn J. Impact of vitamin C on the development, differentiation and functional properties of T cells. Eur J Microbiol Immunol (Bp). 2024;14(2):67-74 https://doi.org/10.1556/1886.2024.00017.
https://doi.org/10.1556/1886.2024.00017 |
| 89 | Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51(4):301-323 https://doi.org/10.1159/000107673.
https://doi.org/10.1159/000107673 |
| 90 | Qvist I, Abdulla M, Mathur A, Robertson B, Svensson S. Zinc, copper, magnesium, and calcium in blood and plasma after phlebotomy. Scand J Haematol. 1983;31(2):161-167 https://doi.org/10.1111/j.1600-0609.1983.tb01525.x.
https://doi.org/10.1111/j.1600-0609.1983.tb01525.x |
| 91 | Morse EE, Cable R, Pisciotto P, Kakaiya R, Kiraly T. Evaluation of iron status in women identified by copper sulfate screening as ineligible to donate blood. Transfusion. 1987;27(3):238-241 https://doi.org/10.1046/j.1537-2995.1987.27387235628.x.
https://doi.org/10.1046/j.1537-2995.1987.27387235628.x |
| 92 | Cable RG, Morse EE, Keltonic J, Kakaiya R, Kiraly T. Iron supplementation in female blood donors deferred by copper sulfate screening. Transfusion. 1988;28(5):422-426 https://doi.org/10.1046/j.1537-2995.1988.28588337328.x.
https://doi.org/10.1046/j.1537-2995.1988.28588337328.x |
| 93 | Hallberg L, Rossander-Hultén L. Iron requirements in menstruating women. Am J Clin Nutr. 1991;54(6):1047-1058 https://doi.org/10.1093/ajcn/54.6.1047.
https://doi.org/10.1093/ajcn/54.6.1047 |
| 94 | Gudjoncik A, Guenancia C, Zeller M, Cottin Y, Vergely C, Rochette L. Iron, oxidative stress, and redox signaling in the cardiovascular system. Mol Nutr Food Res. 2014;58(8):1721-1738 https://doi.org/10.1002/mnfr.201400036.
https://doi.org/10.1002/mnfr.201400036 |
| 95 | Warsch S, Byrnes J. Emerging causes of iron deficiency anemia refractory to oral iron supplementation. World J Gastrointest Pharmacol Ther. 2013;4(3):49-53 https://doi.org/10.4292/wjgpt.v4.i3.49.
https://doi.org/10.4292/wjgpt.v4.i3.49 |
| 96 | Wiersum-Osselton J, Prinsze F, van den Brekel E, van Dongen A, Hermans F, Bokhorst A, der Kreek TM. An intervention study for the prevention of vasovagal reactions and evaluating donors' experience: Analysis of donors' return for subsequent donation. Vox Sang. 2022;117(3):313-320 https://doi.org/ 10.1111/vox.13196.
https://doi.org/10.1111/vox.13196 |
| 97 | Hallberg L. Advantages and disadvantages of an iron-rich diet. Eur J Clin Nutr. 2002;56 Suppl 1:S12-S18 https://doi.org/10.1038/sj.ejcn.1601348.
https://doi.org/10.1038/sj.ejcn.1601348 |
| 98 | Osei Bonsu E, Addo IY, Boadi C, Boadu EF, Okeke SR. Determinants of iron-rich food deficiency among children under 5 years in sub-Saharan Africa: a comprehensive analysis of Demographic and Health Surveys. BMJ Open. 2024;14(3):e079856 https://doi.org/10.1136/bmjopen-2023-079856.
https://doi.org/10.1136/bmjopen-2023-079856 |
| 99 | Lynch SR, Cook JD. Interaction of vitamin C and iron. Ann N Y Acad Sci. 1980;355:32-44 https://doi.org/10.1111/j.1749-6632.1980.tb21325.x.
https://doi.org/10.1111/j.1749-6632.1980.tb21325.x |
| 100 | Spencer BR, White JL, Patel EU, Goel R, Bloch EM, Tobian AA. Eligibility Considerations for Female Whole Blood Donors: Hemoglobin Levels and Iron Status in a Nationally Representative Population. Transfus Med Rev. 2023;37(1):27-35 https://doi.org/10.1016/j.tmrv.2022.11.001.
https://doi.org/10.1016/j.tmrv.2022.11.001 |
| 101 | Reddy KV, Shastry S, Raturi M, Baliga BP. Impact of Regular Whole-Blood Donation on Body Iron Stores. Transfus Med Hemother. 2020;47(1):75-79 https://doi.org/10.1159/000499768.
https://doi.org/10.1159/000499768 |
| 102 | Outten FW, Theil EC. Iron-based redox switches in biology. Antioxid Redox Signal. 2009;11(5):1029-1046 https://doi.org/10.1089/ars.2008.2296.
https://doi.org/10.1089/ars.2008.2296 |
| 103 | Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19(2):164-174.
|
| 104 | Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60-72 https://doi.org/10.2478/intox-2014-0009.
https://doi.org/10.2478/intox-2014-0009 |
| 105 | Meneghini R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic Biol Med. 1997;23(5):783-792 https://doi.org/10.1016/s0891-5849(97)00016-6.
https://doi.org/10.1016/S0891-5849(97)00016-6 |
| 106 | Silvestri L, Pettinato M, Furiosi V, Bavuso Volpe L, Nai A, Pagani A. Managing the Dual Nature of Iron to Preserve Health. Int J Mol Sci. 2023;24(4):3995 https://doi.org/10.3390/ijms24043995.
https://doi.org/10.3390/ijms24043995 |
| 107 | Pantopoulos K, Porwal SK, Tartakoff A, Devireddy L. Mechanisms of mammalian iron homeostasis. Biochemistry. 2012;51(29):5705-5724 https://doi.org/10.1021/bi300752r.
https://doi.org/10.1021/bi300752r |
| 108 | Zhao Z. Iron and oxidizing species in oxidative stress and Alzheimer's disease. Aging Med (Milton). 2019;2(2):82-87 https://doi.org/10.1002/agm2.12074.
https://doi.org/10.1002/agm2.12074 |
| 109 | Allakhverdiev ES, Maksimov GV, Rodnenkov OV. Effect of Dinitrosyl Iron Complex on Albumin Conformation. Biochemistry (Mosc). 2021;86(5):533-539 https://doi.org/10.1134/S0006297921050023.
https://doi.org/10.1134/S0006297921050023 |
| 110 | Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174-1194 https://doi.org/10.1016/j.freeradbiomed.2013.09.001.
https://doi.org/10.1016/j.freeradbiomed.2013.09.001 |
| 111 | Puliyel M, Mainous AG 3rd, Berdoukas V, Coates TD. Iron toxicity and its possible association with treatment of Cancer: lessons from hemoglobinopathies and rare, transfusion-dependent anemias. Free Radic Biol Med. 2015;79:343-351. https://doi.org/10.1016/j.freeradbiomed.2014.10.861
https://doi.org/10.1016/j.freeradbiomed.2014.10.861 |
| 112 | Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9-17 https://doi.org/10.1038/nchembio.1416.
https://doi.org/10.1038/nchembio.1416 |
| 113 | Latunde-Dada GO. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861(8):1893-1900 https://doi.org/10.1016/j.bbagen.2017.05.019.
https://doi.org/10.1016/j.bbagen.2017.05.019 |
| 114 | Truzzi DR, Augusto O, Iretskii AV, Ford PC. Dynamics of Dinitrosyl Iron Complex (DNIC) Formation with Low Molecular Weight Thiols. Inorg Chem. 2019;58(19):13446-13456 https://doi.org/10.1021/acs.inorgchem.9b02338.
https://doi.org/10.1021/acs.inorgchem.9b02338 |
| 115 | Hou L, Zhao M, Huang C, Wu X, Zhang J. Nitric Oxide Improves the Tolerance of Pleurotus ostreatus to Heat Stress by Inhibiting Mitochondrial Aconitase. Appl Environ Microbiol. 2020;86(5):e02303-19 https://doi.org/10.1128/AEM.02303-19.
https://doi.org/10.1128/AEM.02303-19 |
| 116 | Cho SL, Liao CJ, Lu TT. Synthetic methodology for preparation of dinitrosyl iron complexes. J Biol Inorg Chem. 2019;24(4):495-515 https://doi.org/10.1007/s00775-019-01668-z.
https://doi.org/10.1007/s00775-019-01668-z |
| 117 | Fierro FA, Kalomoiris S, Sondergaard CS, Nolta JA. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells. 2011;29(11):1727-1737 https://doi.org/10.1002/stem.720.
https://doi.org/10.1002/stem.720 |
| 118 | Tian Z, Yu T, Liu J, Wang T, Higuchi A. Introduction to stem cells. Prog Mol Biol Transl Sci. 2023;199:3-32 https://doi.org/10.1016/bs.pmbts.2023.02.012.
https://doi.org/10.1016/bs.pmbts.2023.02.012 |
| 119 | Moras M, Lefevre SD, Ostuni MA. From Erythroblasts to Mature Red Blood Cells: Organelle Clearance in Mammals. Front Physiol. 2017;8:1076 https://doi.org/10.3389/fphys.2017.01076.
https://doi.org/10.3389/fphys.2017.01076 |
| 120 | Eisele AS, Cosgrove J, Magniez A, Tubeuf E, Tenreira Bento S, Conrad C, Cayrac F, Tak T, Lyne AM, Urbanus J, Perié L. Erythropoietin directly remodels the clonal composition of murine hematopoietic multipotent progenitor cells. Elife. 2022;11:e66922 https://doi.org/10.7554/eLife.66922.
https://doi.org/10.7554/eLife.66922 |
| 121 | Bhoopalan SV, Huang LJ, Weiss MJ. Erythropoietin regulation of red blood cell production: from bench to bedside and back. F1000Res. 2020;9:F1000 Faculty Rev-1153 https://doi.org/10.12688/f1000research.26648.1.
https://doi.org/10.12688/f1000research.26648.1 |
| 122 | Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14(5):302-314 https://doi.org/10.1038/nri3660.
https://doi.org/10.1038/nri3660 |
| 123 | Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637-650 https://doi.org/10.1016/j.immuni.2011.05.006.
https://doi.org/10.1016/j.immuni.2011.05.006 |
| 124 | Mirantes C, Passegué E, Pietras EM. Pro-inflammatory cytokines: emerging players regulating HSC function in normal and diseased hematopoiesis. Exp Cell Res. 2014;329(2):248-254 https://doi.org/10.1016/j.yexcr.2014.08.017.
https://doi.org/10.1016/j.yexcr.2014.08.017 |
| 125 | Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303-320 https://doi.org/10.1038/s41580-019-0103-9.
https://doi.org/10.1038/s41580-019-0103-9 |
| 126 | Al-Hazimi A. Effect of stress on immunity: a study among healthy blood donors at King Abdul Aziz University Hospital, Jeddah. Ann Saudi Med. 2004;24(1):52-54 https://doi.org/10.5144/0256-4947.2004.52.
https://doi.org/10.5144/0256-4947.2004.52 |
| 127 | Dean L. Blood Groups and Red Cell Antigens [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2005. Chapter 3, Blood transfusions and the immune system. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2265/
|
| 128 | Vazquez MI, Catalan-Dibene J, Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine. 2015;74(2):318-326 https://doi.org/10.1016/j.cyto.2015.02.007.
https://doi.org/10.1016/j.cyto.2015.02.007 |
| 129 | Yunce M, Erdamar H, Bayram NA, Gok S. One more health benefit of blood donation: reduces acute-phase reactants, oxidants and increases antioxidant capacity. J Basic Clin Physiol Pharmacol. 2016;27(6):653-657 https://doi.org/10.1515/jbcpp-2015-0111.
https://doi.org/10.1515/jbcpp-2015-0111 |
| 130 | Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792 https://doi.org/10.1155/2013/956792.
https://doi.org/10.1155/2013/956792 |
| 131 | Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med. 2014;20(7):709-711 https://doi.org/10.1038/nm.3624.
https://doi.org/10.1038/nm.3624 |
| 132 | Quee FA, Peffer K, Ter Braake AD, Van den Hurk K. Cardiovascular Benefits for Blood Donors? A Systematic Review. Transfus Med Rev. 2022;36(3):143-151 https://doi.org/10.1016/j.tmrv.2022.04.004.
https://doi.org/10.1016/j.tmrv.2022.04.004 |
| 133 | Sebastião CS, Sacomboio E, Francisco NM, Paixão J, Cassinela EK, de Vasconcelos JN, Pimentel V, Morais J. Demographic characteristics and risk factors related to high blood pressure among healthy blood donors from Luanda, Angola: A retrospective study. Health Sci Rep. 2023;6(6):e1300 https://doi.org/10.1002/hsr2.1300.
https://doi.org/10.1002/hsr2.1300 |
| 134 | Garrioch MA. The body's response to blood loss. Vox Sang. 2004;87 Suppl1:74-76 https://doi.org/10.1111/j.1741-6892.2004.00435.x.
https://doi.org/10.1111/j.1741-6892.2004.00435.x |
| 135 | Rosa-Bray M, Wisdom C, Marier JF, Mouksassi MS, Wada S. The effect of plasmapheresis on blood pressure in voluntary plasma donors. Vox Sang. 2015;108(1):11-17 https://doi.org/10.1111/vox.12188.
https://doi.org/10.1111/vox.12188 |
| 136 | Srivastava T, Hariharan S, Alon US, McCarthy ET, Sharma R, El-Meanawy A, Savin VJ, Sharma M. Hyperfiltration-mediated Injury in the Remaining Kidney of a Transplant Donor. Transplantation. 2018;102(10):1624-1635 https://doi.org/10.1097/TP.0000000000002304.
https://doi.org/10.1097/TP.0000000000002304 |
| 137 | Ames MK, Atkins CE, Pitt B. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med. 2019;33(2):363-382 https://doi.org/10.1111/jvim.15454.
https://doi.org/10.1111/jvim.15454 |
| 138 | Diab A, Dastmalchi LN, Gulati M, Michos ED. A Heart-Healthy Diet for Cardiovascular Disease Prevention: Where Are We Now?. Vasc Health Risk Manag. 2023;19:237-253 https://doi.org/10.2147/VHRM.S379874.
https://doi.org/10.2147/VHRM.S379874 |
| 139 | Roumelioti ME, Glew RH, Khitan ZJ, Rondon-Berrios H, Argyropoulos CP, Malhotra D, Raj DS, Agaba EI, Rohrscheib M, Murata GH, Shapiro JI, Tzamaloukas AH. Fluid balance concepts in medicine: Principles and practice. World J Nephrol. 2018;7(1):1-28 https://doi.org/10.5527/wjn.v7.i1.1.
https://doi.org/10.5527/wjn.v7.i1.1 |
| 140 | Peffer K, den Heijer M, de Kort WLAM, Verbeek ALM, Atsma F. Cardiovascular risk in 159 934 frequent blood donors while addressing the healthy donor effect. Heart. 2019;105(16):1260-1265 https://doi.org/10.1136/heartjnl-2018-314138.
https://doi.org/10.1136/heartjnl-2018-314138 |
| 141 | Kris-Etherton PM, Petersen KS, Hibbeln JR, Hurley D, Kolick V, Peoples S, Rodriguez N, Woodward-Lopez G. Nutrition and behavioral health disorders: depression and anxiety. Nutr Rev. 2021;79(3):247-260 https://doi.org/10.1093/nutrit/nuaa025.
https://doi.org/10.1093/nutrit/nuaa025 |
| 142 | Peffer K, de Kort WL, Slot E, Doggen CJ. Turbid plasma donations in whole blood donors: fat chance?. Transfusion. 2011;51(6):1179-1187 https://doi.org/10.1111/j.1537-2995.2011.03079.x.
https://doi.org/10.1111/j.1537-2995.2011.03079.x |
| 143 | Vassallo RR, Stearns FM. Lipemic plasma: a renaissance. Transfusion. 2011;51(6):1136-1139 https://doi.org/10.1111/j.1537-2995.2011.03142.x.
https://doi.org/10.1111/j.1537-2995.2011.03142.x |
| 144 | Krasowski MD. Educational Case: Hemolysis and Lipemia Interference With Laboratory Testing. Acad Pathol. 2019;6:2374289519888754 https://doi.org/10.1177/2374289519888754.
https://doi.org/10.1177/2374289519888754 |
| 145 | Fernández Prendes C, Castro Castro MJ, Sánchez Navarro L, Rapún Mas L, Morales Indiano C, Arrobas Velilla T. Handling of lipemic samples in the clinical laboratory. Adv Lab Med. 2023;4(1):5-27 https://doi.org/10.1515/almed-2023-0003.
https://doi.org/10.1515/almed-2023-0003 |
| 146 | Lippi G, Franchini M. Lipaemic donations: truth and consequences. Transfus Apher Sci. 2013;49(2):181-184 https://doi.org/10.1016/j.transci.2012.10.001.
https://doi.org/10.1016/j.transci.2012.10.001 |
| 147 | Bashir S, Wiltshire M, Cardigan R, Thomas S. Lipaemic plasma induces haemolysis in resuspended red cell concentrate. Vox Sang. 2013;104(3):218-224 https://doi.org/10.1111/j.1423-0410.2012.01660.x.
https://doi.org/10.1111/j.1423-0410.2012.01660.x |
| 148 | Winter KM, Webb RG, Marks DC. Red cells manufactured from lipaemic whole blood donations: Do they have higher haemolysis?. Vox Sang. 2022;117(12):1351-1359 https://doi.org/10.1111/vox.13366.
https://doi.org/10.1111/vox.13366 |
| 149 | Ems T, St Lucia K, Huecker MR. Biochemistry, Iron Absorption [Updated 2023 Apr 17].. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448204/.
|
| 150 | Hurrell RF, Trinidad TP, Mallillin AC, Sagum RS, Foman JT, Li Q, Zeder C, Kastenmayer P, Rytz A, Sabatier M, Egli I. Iron Bioavailability from Ferrous Ammonium Phosphate, Ferrous Sulfate, and Ferric Pyrophosphate in an Instant Milk Drink-A Stable Isotope Study in Children. Nutrients. 2022;14(8):1640 https://doi.org/10.3390/nu14081640.
https://doi.org/10.3390/nu14081640 |
| 151 | Man Y, Xu T, Adhikari B, Zhou C, Wang Y, Wang B. Iron supplementation and iron-fortified foods: a review. Crit Rev Food Sci Nutr. 2022;62(16):4504-4525 https://doi.org/10.1080/10408398.2021.1876623.
https://doi.org/10.1080/10408398.2021.1876623 |
| 152 | Hunt JR. Dietary and physiological factors that affect the absorption and bioavailability of iron. Int J Vitam Nutr Res. 2005;75(6):375-384 https://doi.org/10.1024/0300-9831.75.6.375.
https://doi.org/10.1024/0300-9831.75.6.375 |
| 153 | Carpenter CE, Mahoney AW. Contributions of heme and nonheme iron to human nutrition. Crit Rev Food Sci Nutr. 1992;31(4):333-367 https://doi.org/10.1080/10408399209527576.
https://doi.org/10.1080/10408399209527576 |
| 154 | Hallberg L, Hoppe M, Andersson M, Hulthén L. The role of meat to improve the critical iron balance during weaning. Pediatrics. 2003;111(4 Pt 1):864-870 https://doi.org/10.1542/peds.111.4.864.
https://doi.org/10.1542/peds.111.4.864 |
| 155 | Moll R, Davis B. Iron, vitamin B12 and folate. Medicine. 2017;45(4):198-203 https://doi.org/10.1016/j.mpmed.2017.01.007.
https://doi.org/10.1016/j.mpmed.2017.01.007 |
| 156 | Vogt AS, Arsiwala T, Mohsen M, Vogel M, Manolova V, Bachmann MF. On Iron Metabolism and Its Regulation. Int J Mol Sci. 2021;22(9):4591 https://doi.org/10.3390/ijms22094591.
https://doi.org/10.3390/ijms22094591 |
| 157 | Riško P, Pláteník J, Buchal R, Potočková J, Kraml PJ. Long-term donors versus non-donor men: Iron metabolism and the atherosclerotic process. Atherosclerosis. 2018;272:14-20 https://doi.org/10.1016/j.atherosclerosis.2018.03.009.
https://doi.org/10.1016/j.atherosclerosis.2018.03.009 |
| 158 | Suntres ZE. Liposomal Antioxidants for Protection against Oxidant-Induced Damage. J Toxicol. 2011;2011:152474 https://doi.org/10.1155/2011/152474.
https://doi.org/10.1155/2011/152474 |
| 159 | Tzounakas VL, Anastasiadi AT, Arvaniti VZ. Supplementation with uric and ascorbic acid protects stored red blood cells through enhancement of non-enzymatic antioxidant activity and metabolic rewiring. Redox Biol. 2022;57:102477 https://doi.org/10.1016/j.redox.2022.102477.
https://doi.org/10.1016/j.redox.2022.102477 |
| 160 | Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51(5):1000-1013 https://doi.org/10.1016/j.freeradbiomed.2011.05.017.
https://doi.org/10.1016/j.freeradbiomed.2011.05.017 |
| 161 | Pizzorno J. Glutathione!. Integr Med (Encinitas). 2014;13(1):8-12.
|
| 162 | Lee E, Park HY, Kim SW, Kim J, Lim K. Vitamin C and glutathione supplementation: a review of their additive effects on exercise performance. Phys Act Nutr. 2023;27(3):36-43 https://doi.org/10.20463/pan.2023.0027.
https://doi.org/10.20463/pan.2023.0027 |
| 163 | Pallavi M, Rajashekaraiah V. Synergistic activity of vitamin-C and vitamin-E to ameliorate the efficacy of stored erythrocytes. Transfus Clin Biol. 2023;30(1):87-95 https://doi.org/10.1016/j.tracli.2022.09.002.
https://doi.org/10.1016/j.tracli.2022.09.002 |
| 164 | Theodosis-Nobelos P, Papagiouvannis G, Tziona P, Rekka EA. Lipoic acid. Kinetics and pluripotent biological properties and derivatives. Mol Biol Rep. 2021;48(9):6539-6550 https://doi.org/10.1007/s11033-021-06643-z.
https://doi.org/10.1007/s11033-021-06643-z |
| 165 | Mantle D, Turton N, Hargreaves IP. Depletion and Supplementation of Coenzyme Q10 in Secondary Deficiency Disorders. Front Biosci (Landmark Ed). 2022;27(12):322 https://doi.org/10.31083/j.fbl2712322.
https://doi.org/10.31083/j.fbl2712322 |
| 166 | Du LL, Fu QY, Xiang LP, Zheng XQ, Lu JL, Ye JH, Li QS, Polito CA, Liang YR. Tea Polysaccharides and Their Bioactivities. Molecules. 2016;21(11):1449 https://doi.org/10.3390/molecules21111449.
https://doi.org/10.3390/molecules21111449 |
| 167 | Zhang MY, Malins LR. Decarboxylative Couplings for Late-Stage Peptide Modifications. Methods Mol Biol. 2020;2103:275-285 https://doi.org/10.1007/978-1-0716-0227-0_19.
https://doi.org/10.1007/978-1-0716-0227-0_19 |
| 168 | Wani AL, Bhat SA, Ara A. Omega-3 fatty acids and the treatment of depression: a review of scientific evidence. Integr Med Res. 2015;4(3):132-141 https://doi.org/10.1016/j.imr.2015.07.003.
https://doi.org/10.1016/j.imr.2015.07.003 |
| 169 | Friedman M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int J Tryptophan Res. 2018;11:1178646918802282 https://doi.org/10.1177/1178646918802282.
https://doi.org/10.1177/1178646918802282 |
| 170 | Noah L, Dye L, Bois De Fer B, Mazur A, Pickering G, Pouteau E. Effect of magnesium and vitamin B6 supplementation on mental health and quality of life in stressed healthy adults: Post-hoc analysis of a randomised controlled trial. Stress Health. 2021;37(5):1000-1009 https://doi.org/10.1002/smi.3051.
https://doi.org/10.1002/smi.3051 |
| 171 | Pereira GA, da Silva A, Hermsdorff HHM, Moreira APB, de Aguiar AS. Association of dietary total antioxidant capacity with depression, anxiety, and sleep disorders: A systematic review of observational studies. J Clin Transl Res. 2021;7(5):631-640.
|
| 172 | Garcia-Garcia D. Health Promotion and Hydration: A Systematic Review About Hydration Care. Florence Nightingale J Nurs. 2022;30(3):310-321 https://doi.org/10.5152/FNJN.2022.21313.
https://doi.org/10.5152/FNJN.2022.21313 |
| 173 | Milto IV, Suhodolo IV, Prokopieva VD, Klimenteva TK. Molecular and Cellular Bases of Iron Metabolism in Humans. Biochemistry (Mosc). 2016;81(6):549-564 https://doi.org/10.1134/S0006297916060018.
https://doi.org/10.1134/S0006297916060018 |
| 174 | Duck KA, Connor JR. Iron uptake and transport across physiological barriers. Biometals. 2016;29(4):573-591 https://doi.org/10.1007/s10534-016-9952-2.
https://doi.org/10.1007/s10534-016-9952-2 |
| 175 | Srai SK, Bomford A, McArdle HJ. Iron transport across cell membranes: molecular understanding of duodenal and placental iron uptake. Best Pract Res Clin Haematol. 2002;15(2):243-259 https://doi.org/10.1016/s1521-6926(02)90003-4.
https://doi.org/10.1016/S1521-6926(02)90003-4 |
| 176 | Dutt S, Hamza I, Bartnikas TB. Molecular Mechanisms of Iron and Heme Metabolism. Annu Rev Nutr. 2022;42:311-335 https://doi.org/10.1146/annurev-nutr-062320-112625.
https://doi.org/10.1146/annurev-nutr-062320-112625 |
| 177 | Porter JB. Concepts and goals in the management of transfusional iron overload. Am J Hematol. 2007;82(12 Suppl):1136-1139 https://doi.org/10.1002/ajh.21100.
https://doi.org/10.1002/ajh.21100 |
| 178 | Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88(1):7-15 https://doi.org/10.1007/s12185-008-0120-5.
https://doi.org/10.1007/s12185-008-0120-5 |
| 179 | Kakhlon O, Breuer W, Munnich A, Cabantchik ZI. Iron redistribution as a therapeutic strategy for treating diseases of localized iron accumulation. Can J Physiol Pharmacol. 2010;88(3):187-196 https://doi.org/10.1139/Y09-128.
https://doi.org/10.1139/Y09-128 |
| 180 | Fibach E, Rachmilewitz EA. Iron overload in hematological disorders. Presse Med. 2017;46(12 Pt 2):e296-e305 https://doi.org/10.1016/j.lpm.2017.10.007.
https://doi.org/10.1016/j.lpm.2017.10.007 |
| 181 | Conrad ME, Umbreit JN, Moore EG, Heiman D. Mobilferrin is an intermediate in iron transport between transferrin and hemoglobin in K562 cells. J Clin Invest. 1996;98(6):1449-1454 https://doi.org/10.1172/JCI118933.
https://doi.org/10.1172/JCI118933 |
| 182 | Yanatori I, Kishi F. DMT1 and iron transport. Free Radic Biol Med. 2019;133:55-63 https://doi.org/10.1016/j.freeradbiomed.2018.07.020.
https://doi.org/10.1016/j.freeradbiomed.2018.07.020 |
| 183 | Ianiro G, Rosa L, Bonaccorsi di Patti MC, Valenti P, Musci G, Cutone A. Lactoferrin: from the structure to the functional orchestration of iron homeostasis. Biometals. 2023;36(3):391-416 https://doi.org/10.1007/s10534-022-00453-x.
https://doi.org/10.1007/s10534-022-00453-x |
| 184 | Perera DN, Palliyaguruge CL, Eapasinghe DD, Liyanage DM, Seneviratne RACH, Demini SMD, Jayasinghe JASM, Faizan M, Rajagopalan U, Galhena BP, Hays H, Senathilake K, Tennekoon KH, Samarakoon SR. Factors affecting iron absorption and the role of fortification in enhancing iron levels. Nutr Bull. 2023;48(4):442-457 https://doi.org/10.1111/nbu.12643.
https://doi.org/10.1111/nbu.12643 |
| 185 | Kell DB, Heyden EL, Pretorius E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front Immunol. 2020;11:1221 https://doi.org/10.3389/fimmu.2020.01221.
https://doi.org/10.3389/fimmu.2020.01221 |
| 186 | Luck AN, Mason AB. Transferrin-mediated cellular iron delivery. Curr Top Membr. 2012;69:3-35 https://doi.org/10.1016/B978-0-12-394390-3.00001-X.
https://doi.org/10.1016/B978-0-12-394390-3.00001-X |
| 187 | Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr. 2017;106(Suppl 6):1559S-1566S. https://doi.org/10.3945/ajcn.117.155804.
https://doi.org/10.3945/ajcn.117.155804 |
| 188 | Papanikolaou G, Pantopoulos K. Systemic iron homeostasis and erythropoiesis. IUBMB Life. 2017;69(6):399-413 https://doi.org/10.1002/iub.1629.
https://doi.org/10.1002/iub.1629 |
| 189 | Gozzelino R, Arosio P. Iron Homeostasis in Health and Disease. Int J Mol Sci. 2016;17(1):130 https://doi.org/10.3390/ijms17010130.
https://doi.org/10.3390/ijms17010130 |
| 190 | Camaschella C. Iron and hepcidin: a story of recycling and balance. Hematology Am Soc Hematol Educ Program. 2013;2013:1-8 https://doi.org/10.1182/asheducation-2013.1.1.
https://doi.org/10.1182/asheducation-2013.1.1 |
| 191 | Fleming MD. The regulation of hepcidin and its effects on systemic and cellular iron metabolism. Hematology Am Soc Hematol Educ Program. 2008;151-158 https://doi.org/10.1182/asheducation-2008.1.151.
https://doi.org/10.1182/asheducation-2008.1.151 |
| 192 | Kaplan J, Ward DM, De Domenico I. The molecular basis of iron overload disorders and iron-linked anemias. Int J Hematol. 2011;93(1):14-20 https://doi.org/10.1007/s12185-010-0760-0.
https://doi.org/10.1007/s12185-010-0760-0 |
| 193 | De Domenico I, Ward DM, Kaplan J. Hepcidin and ferroportin: the new players in iron metabolism. Semin Liver Dis. 2011;31(3):272-279 https://doi.org/10.1055/s-0031-1286058.
https://doi.org/10.1055/s-0031-1286058 |
| 194 | Hoppe M, Brün B, Larsson MP, Moraeus L, Hulthén L. Heme iron-based dietary intervention for improvement of iron status in young women. Nutrition. 2013;29(1):89-95 https://doi.org/10.1016/j.nut.2012.04.013.
https://doi.org/10.1016/j.nut.2012.04.013 |
| 195 | Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39 https://doi.org/10.1182/blood-2018-05-815944.
https://doi.org/10.1182/blood-2018-05-815944 |
| 196 | Kumar A, Sharma E, Marley A, Samaan MA, Brookes MJ. Iron deficiency anaemia: pathophysiology, assessment, practical management. BMJ Open Gastroenterol. 2022;9(1):e000759 https://doi.org/10.1136/bmjgast-2021-000759.
https://doi.org/10.1136/bmjgast-2021-000759 |
| 197 | Weckmann G, Kiel S, Chenot JF, Angelow A. Association of Anemia with Clinical Symptoms Commonly Attributed to Anemia-Analysis of Two Population-Based Cohorts. J Clin Med. 2023;12(3):921 https://doi.org/10.3390/jcm12030921.
https://doi.org/10.3390/jcm12030921 |
| 198 | Holbein BE, Lehmann C. Dysregulated Iron Homeostasis as Common Disease Etiology and Promising Therapeutic Target. Antioxidants (Basel). 2023;12(3):671 https://doi.org/10.3390/antiox12030671.
https://doi.org/10.3390/antiox12030671 |
| 199 | Dziegala M, Josiak K, Kasztura M. Iron deficiency as energetic insult to skeletal muscle in chronic diseases. J Cachexia Sarcopenia Muscle. 2018;9(5):802-815 https://doi.org/10.1002/jcsm.12314.
https://doi.org/10.1002/jcsm.12314 |
| 200 | Stugiewicz M, Tkaczyszyn M, Kasztura M, Banasiak W, Ponikowski P, Jankowska EA. The influence of iron deficiency on the functioning of skeletal muscles: experimental evidence and clinical implications. Eur J Heart Fail. 2016;18(7):762-773 https://doi.org/10.1002/ejhf.467.
https://doi.org/10.1002/ejhf.467 |
| 201 | Stein J, Connor S, Virgin G, Ong DE, Pereyra L. Anemia and iron deficiency in gastrointestinal and liver conditions. World J Gastroenterol. 2016;22(35):7908-7925 https://doi.org/10.3748/wjg.v22.i35.7908.
https://doi.org/10.3748/wjg.v22.i35.7908 |
| 202 | Carabotti M, Annibale B, Lahner E. Common Pitfalls in the Management of Patients with Micronutrient Deficiency: Keep in Mind the Stomach. Nutrients. 2021;13(1):208 https://doi.org/10.3390/nu13010208.
https://doi.org/10.3390/nu13010208 |
| 203 | Rawee P, Kremer D, Nolte IM, Leuvenink HGD, Touw DJ, De Borst MH, Bakker SJL, Hanudel MR, Eisenga MF. Iron Deficiency and Nephrotoxic Heavy Metals: A Dangerous Interplay?. Int J Mol Sci. 2023;24(6):5315 https://doi.org/10.3390/ijms24065315.
https://doi.org/10.3390/ijms24065315 |
| 204 | Kim Y. Effect of Iron Deficiency on the Increased Blood Divalent Metal Concentrations [Internet]. Iron Deficiency Anemia. IntechOpen 2019. Available from: http://dx.doi.org/10.5772/intechopen.78958.
https://doi.org/10.5772/intechopen.78958 |
| 205 | Nairz M, Weiss G. Iron in infection and immunity. Mol Aspects Med. 2020;75:100864 https://doi.org/10.1016/j.mam.2020.100864.
https://doi.org/10.1016/j.mam.2020.100864 |
| 206 | Frost JN, Wideman SK, Preston AE, Teh MR, Ai Z, Wang L, Cross A, White N, Yazicioglu Y, Bonadonna M, Clarke AJ, Armitage AE, Galy B, Udalova IA, Drakesmith H. Plasma iron controls neutrophil production and function. Sci Adv. 2022;8(40):eabq5384 https://doi.org/10.1126/sciadv.abq5384.
https://doi.org/10.1126/sciadv.abq5384 |
| 207 | Abuga KM, Nairz M, MacLennan CA, Atkinson SH. Severe anaemia, iron deficiency, and susceptibility to invasive bacterial infections. Wellcome Open Res. 2023;8:48 https://doi.org/10.12688/wellcomeopenres.18829.1.
https://doi.org/10.12688/wellcomeopenres.18829.1 |
| 208 | Hurrell RF. Bioavailability of iron. Eur J Clin Nutr. 1997;51 Suppl 1:S4-S8.
|
| 209 | Moretti D, Zimmermann MB, Wegmüller R, Walczyk T, Zeder C, Hurrell RF. Iron status and food matrix strongly affect the relative bioavailability of ferric pyrophosphate in humans. Am J Clin Nutr. 2006;83(3):632-638 https://doi.org/10.1093/ajcn.83.3.632.
https://doi.org/10.1093/ajcn.83.3.632 |
| 210 | Hurrell I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91(5):1461S-1467S. https://doi.org/10.3945/ajcn.2010.28674F.
https://doi.org/10.3945/ajcn.2010.28674F |
| 211 | Bothwell TH, MacPhail AP. The potential role of NaFeEDTA as an iron fortificant. Int J Vitam Nutr Res. 2004;74(6):421-434 https://doi.org/10.1024/0300-9831.74.6.421.
https://doi.org/10.1024/0300-9831.74.6.421 |
| 212 | Watanapaisantrakul R, Chavasit V, Kongkachuichai R. Fortification of soy sauce using various iron sources: sensory acceptability and shelf stability. Food Nutr Bull. 2006;27(1):19-25 https://doi.org/10.1177/156482650602700103.
https://doi.org/10.1177/156482650602700103 |
| 213 | Mellican RI, Li J, Mehansho H, Nielsen SS. The role of iron and the factors affecting off-color development of polyphenols. J Agric Food Chem. 2003;51(8):2304-2316 https://doi.org/10.1021/jf020681c.
https://doi.org/10.1021/jf020681c |
| 214 | Henare SJ, Nur Singh N, Ellis AM, Moughan PJ, Thompson AK, Walczyk T. Iron bioavailability of a casein-based iron fortificant compared with that of ferrous sulfate in whole milk: a randomized trial with a crossover design in adult women. Am J Clin Nutr. 2019;110(6):1362-1369 https://doi.org/10.1093/ajcn/nqz237.
https://doi.org/10.1093/ajcn/nqz237 |
| 215 | Kumari A, Chauhan AK. Iron nanoparticles as a promising compound for food fortification in iron deficiency anemia: a review. J Food Sci Technol. 2022;59(9):3319-3335 https://doi.org/10.1007/s13197-021-05184-4.
https://doi.org/10.1007/s13197-021-05184-4 |
| 216 | Ghosh R, Arcot J. Fortification of foods with nano-iron: its uptake and potential toxicity: current evidence, controversies, and research gaps. Nutr Rev. 2022;80(9):1974-1984 https://doi.org/10.1093/nutrit/nuac011.
https://doi.org/10.1093/nutrit/nuac011 |
| 217 | Lynch SR. Interaction of iron with other nutrients. Nutr Rev. 1997;55(4):102-110 https://doi.org/10.1111/j.1753-4887.1997.tb06461.x.
https://doi.org/10.1111/j.1753-4887.1997.tb06461.x |
| 218 | Hurrell RF, Lynch S, Bothwell T, Cori H, Glahn R, Hertrampf E, Kratky Z, Miller D, Rodenstein M, Streekstra H, Teucher B, Turner E, Yeung CK, Zim Hurrell RF, Lynch S, Bothwell T, Cori H, Glahn R, Hertrampf E, Kratky Z, Miller D, Rodenstein M, Streekstra H, Teucher B, Turner E, Yeung CK, Zimmermann MB. SUSTAIN Task Force. Enhancing the absorption of fortification iron. A SUSTAIN Task Force report. Int J Vitam Nutr Res. 2004;74(6):387-401 https://doi.org/ 10.1024/0300-9831.74.6.387.
https://doi.org/10.1024/0300-9831.74.6.387 |
| 219 | Kim HS, Quon MJ, Kim JA. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187-195 https://doi.org/10.1016/j.redox.2013.12.022.
https://doi.org/10.1016/j.redox.2013.12.022 |
| 220 | Andrews M, Briones L, Jaramillo A, Pizarro F, Arredondo M. Effect of calcium, tannic acid, phytic acid and pectin over iron uptake in an in vitro Caco-2 cell model. Biol Trace Elem Res. 2014;158(1):122-127 https://doi.org/10.1007/s12011-014-9911-0.
https://doi.org/10.1007/s12011-014-9911-0 |
| 221 | Bohn L, Meyer AS, Rasmussen SK. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J Zhejiang Univ Sci B. 2008;9(3):165-191 https://doi.org/10.1631/jzus.B0710640.
https://doi.org/10.1631/jzus.B0710640 |
| 222 | Ma Q, Kim EY, Lindsay EA, Han O. Bioactive dietary polyphenols inhibit heme iron absorption in a dose-dependent manner in human intestinal Caco-2 cells. J Food Sci. 2011;76(5):H143-H150 https://doi.org/10.1111/j.1750-3841.2011.02184.x.
https://doi.org/10.1111/j.1750-3841.2011.02184.x |
| 223 | Kim EY, Ham SK, Shigenaga MK, Han O. Bioactive dietary polyphenolic compounds reduce nonheme iron transport across human intestinal cell monolayers. J Nutr. 2008;138(9):1647-1651 https://doi.org/10.1093/jn/138.9.1647.
https://doi.org/10.1093/jn/138.9.1647 |
| 224 | Huo JS, Yin JY, Sun J, Huang J, Lu ZX, Regina MP, Chen JS, Chen CM: Effect of NaFeEDTA-Fortified Soy Sauce on Anemia Prevalence in China: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Biomed Environ Sci. 2015;28(11):788-798 https://doi.org/10.3967/bes2015.110.
https://doi.org/10.1016/S0895-3988(15)30109-4 |
| 225 | Hurrell RF. Iron Fortification Practices and Implications for Iron Addition to Salt. J Nutr. 2021;151(Suppl 1):3S-14S. https://doi.org/10.1093/jn/nxaa175.
https://doi.org/10.1093/jn/nxaa175 |
| 226 | De-Regil LM, Casanueva E, Killilea DW, Viteri FE. Dialyzability of minerals in corn masa gruel (atole) fortified with different iron compounds: effects of ascorbic acid, disodium EDTA, and phytic acid. Food Nutr Bull. 2007;28(2):198-205 https://doi.org/10.1177/156482650702800209.
https://doi.org/10.1177/156482650702800209 |
| 227 | Fischer JAJ, Cherian AM, Bone JN, Karakochuk CD. The effects of oral ferrous bisglycinate supplementation on hemoglobin and ferritin concentrations in adults and children: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2023;81(8):904-920 https://doi.org/10.1093/nutrit/nuac106.
https://doi.org/10.1093/nutrit/nuac106 |
| 228 | Duque X, Martinez H, Vilchis-Gil J, Mendoza E, Flores-Hernández S, Morán S, Navarro F, Roque-Evangelista V, Serrano A, Mera RM. Effect of supplementation with ferrous sulfate or iron bis-glycinate chelate on ferritin concentration in Mexican schoolchildren: a randomized controlled trial. Nutr J. 2014;13:71 https://doi.org/10.1186/1475-2891-13-71.
https://doi.org/10.1186/1475-2891-13-71 |
| 229 | Bagna R, Spada E, Mazzone R, Saracco P, Boetti T, Cester EA, Bertino E, Coscia A. Efficacy of Supplementation with Iron Sulfate Compared to Iron Bisglycinate Chelate in Preterm Infants. Curr Pediatr Rev. 2018;14(2):123-129 https://doi.org/10.2174/1573396314666180124101059.
https://doi.org/10.2174/1573396314666180124101059 |
| 230 | Piperno A, Pelucchi S, Mariani R. Inherited iron overload disorders. Transl Gastroenterol Hepatol. 2020;5:25 https://doi.org/10.21037/tgh.2019.11.15.
https://doi.org/10.21037/tgh.2019.11.15 |
| 231 | Stremmel W, Lotz G, Niederau C, Teschke R, Strohmeyer G. Iron uptake by rat duodenal microvillous membrane vesicles: evidence for a carrier mediated transport system. Eur J Clin Invest. 1987;17(2):136-145 https://doi.org/10.1111/j.1365-2362.1987.tb02393.x.
https://doi.org/10.1111/j.1365-2362.1987.tb02393.x |
| 232 | Li Y, Jiang H, Huang G. Protein Hydrolysates as Promoters of Non-Haem Iron Absorption. Nutrients. 2017;9(6):609 https://doi.org/10.3390/nu9060609.
https://doi.org/10.3390/nu9060609 |
| 233 | Wallace DF. The Regulation of Iron Absorption and Homeostasis. Clin Biochem Rev. 2016;37(2):51-62.
|
| 234 | Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74(6):403-419 https://doi.org/10.1024/0300-9831.74.6.403.
https://doi.org/10.1024/0300-9831.74.6.403 |
| 235 | Pizarro F, Olivares M, Hertrampf E, Mazariegos DI, Arredondo M, Letelier A, Gidi V. Iron bis-glycine chelate competes for the nonheme-iron absorption pathway. Am J Clin Nutr. 2002;76(3):577-581 https://doi.org/10.1093/ajcn/76.3.577.
https://doi.org/10.1093/ajcn/76.3.577 |
| 236 | Pineda O. Iron bis-glycine chelate competes for the nonheme-iron absorption pathway. Am J Clin Nutr. 2003;78(3):495-496 https://doi.org/10.1093/ajcn/78.3.495.
https://doi.org/10.1093/ajcn/78.3.495 |
| 237 | Kaur N, Agarwal A, Sabharwal M. Food fortification strategies to deliver nutrients for the management of iron deficiency anaemia. Curr Res Food Sci. 2022;5:2094-2107 https://doi.org/10.1016/j.crfs.2022.10.020.
https://doi.org/10.1016/j.crfs.2022.10.020 |
| 238 | Collings R, Harvey LJ, Hooper L, Hurst R, Brown TJ, Ansett J, King M, Fairweather-Tait SJ. The absorption of iron from whole diets: a systematic review. Am J Clin Nutr. 2013;98(1):65-81 https://doi.org/10.3945/ajcn.112.050609.
https://doi.org/10.3945/ajcn.112.050609 |
| 239 | Ahmed MH, Ghatge MS, Safo MK. Hemoglobin: Structure, Function and Allostery. Subcell Biochem. 2020;94:345-382 https://doi.org/10.1007/978-3-030-41769-7_14.
https://doi.org/10.1007/978-3-030-41769-7_14 |
| 240 | Kiss JE. Laboratory and genetic assessment of iron deficiency in blood donors. Clin Lab Med. 2015;35(1):73-91 https://doi.org/10.1016/j.cll.2014.10.011.
https://doi.org/10.1016/j.cll.2014.10.011 |
| 241 | Timmer TC, de Groot R, Rijnhart JJM, Lakerveld J, Brug J, Perenboom CWM, Baart MA, Prinsze FJ, Zalpuri S, van der Schoot EC, de Kort WLAM, van den Hurk K. Dietary intake of heme iron is associated with ferritin and hemoglobin levels in Dutch blood donors: results from Donor InSight. Haematologica. 2020;105(10):2400-2406 https://doi.org/10.3324/haematol.2019.229450.
https://doi.org/10.3324/haematol.2019.229450 |
| 242 | Skolmowska D, Głąbska D. Analysis of Heme and Non-Heme Iron Intake and Iron Dietary Sources in Adolescent Menstruating Females in a National Polish Sample. Nutrients. 2019;11(5):1049 https://doi.org/10.3390/nu11051049.
https://doi.org/10.3390/nu11051049 |
| 243 | Boulton F: Managing donors and iron deficiency. Vox Sang. 2004;87 Suppl 2:22-24 https://doi.org/10.1111/j.1741-6892.2004.00448.x.
https://doi.org/10.1111/j.1741-6892.2004.00448.x |
| 244 | Mast AE, Szabo A, Stone M, Cable RG, Spencer BR, Kiss JE. NHLBI Recipient Epidemiology Donor Evaluation Study (REDS)-III. The benefits of iron supplementation following blood donation vary with baseline iron status. Am J Hematol. 2020;95(7):784-791 https://doi.org/ 10.1002/ajh.25800.
https://doi.org/10.1002/ajh.25800 |
| 245 | Moghadam AM, Natanzi MM, Djalali M, Saedisomeolia A, Javanbakht MH, Saboor-Yaraghi AA, Zareei M. Relationship between blood donors' iron status and their age, body mass index and donation frequency. Sao Paulo Med J. 2013;131(6):377-383 https://doi.org/10.1590/1516-3180.2013.1316554.
https://doi.org/10.1590/1516-3180.2013.1316554 |
| 246 | Lobier M, Castrén J, Niittymäki P, Palokangas E, Partanen J, Arvas M. The effect of donation activity dwarfs the effect of lifestyle, diet and targeted iron supplementation on blood donor iron stores. PLoS One. 2019;14(8):e0220862 https://doi.org/10.1371/journal.pone.0220862.
https://doi.org/10.1371/journal.pone.0220862 |
| 247 | Milman NT. Managing Genetic Hemochromatosis: An Overview of Dietary Measures, Which May Reduce Intestinal Iron Absorption in Persons With Iron Overload. Gastroenterology Res. 2021;14(2):66-80 https://doi.org/10.14740/gr1366.
https://doi.org/10.14740/gr1366 |
| 248 | Briguglio M, Hrelia S, Malaguti M, Lombardi G, Riso P, Porrini M, Perazzo P, Banfi G. The Central Role of Iron in Human Nutrition: From Folk to Contemporary Medicine. Nutrients. 2020;12(6):1761 https://doi.org/10.3390/nu12061761.
https://doi.org/10.3390/nu12061761 |
| 249 | Jackson J, Williams R, McEvoy M, MacDonald-Wicks L, Patterson A. Is Higher Consumption of Animal Flesh Foods Associated with Better Iron Status among Adults in Developed Countries? A Systematic Review. Nutrients. 2016;8(2):89 https://doi.org/10.3390/nu8020089.
https://doi.org/10.3390/nu8020089 |
| 250 | Beck KL, Conlon CA, Kruger R, Coad J. Dietary determinants of and possible solutions to iron deficiency for young women living in industrialized countries: a review. Nutrients. 2014;6(9):3747-3776 https://doi.org/10.3390/nu6093747.
https://doi.org/10.3390/nu6093747 |
| 251 | Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001. 9, Iron. Available from: https://www.ncbi.nlm.nih.gov/books/NBK222309/.
|
| 252 | Lombardi-Boccia G, Lanzi S, Lucarini M, Di Lullo G. Meat and meat products consumption in Italy: contribution to trace elements, heme iron and selected B vitamins supply. Int J Vitam Nutr Res. 2004;74(4):247-251 https://doi.org/10.1024/0300-9831.74.4.247.
https://doi.org/10.1024/0300-9831.74.4.247 |
| 253 | Rigas AS, Sørensen CJ, Pedersen OB, Petersen MS, Thørner LW, Kotzé S, Sørensen E, Magnussen K, Rostgaard K, Erikstrup C, Ullum H. Predictors of iron levels in 14, 737 Danish blood donors: results from the Danish Blood Donor Study. Transfusion. 2014;54(3 Pt 2):789-796 https://doi.org/10.1111/trf.12518.
https://doi.org/10.1111/trf.12518 |
| 254 | Pawlak R, Berger J, Hines I. Iron Status of Vegetarian Adults: A Review of Literature. Am J Lifestyle Med. 2016;12(6):486-498 https://doi.org/10.1177/1559827616682933.
https://doi.org/10.1177/1559827616682933 |
| 255 | Fairweather-Tait SJ. Iron nutrition in the UK: getting the balance right. Proc Nutr Soc. 2004;63(4):519-528 https://doi.org/10.1079/pns2004394.
https://doi.org/10.1079/PNS2004394 |
| 256 | Kontoghiorghes GJ, Kolnagou A, Kontoghiorghe CN, Mourouzidis L, Timoshnikov VA, Polyakov NE. Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications. Medicines (Basel). 2020;7(8):45 https://doi.org/10.3390/medicines7080045.
https://doi.org/10.3390/medicines7080045 |
| 257 | Prentice AM, Mendoza YA, Pereira D. Dietary strategies for improving iron status: balancing safety and efficacy. Nutr Rev. 2017;75(1):49-60 https://doi.org/10.1093/nutrit/nuw055.
https://doi.org/10.1093/nutrit/nuw055 |
| 258 | Bhoot HR, Zamwar UM, Chakole S, Anjankar A. Dietary Sources, Bioavailability, and Functions of Ascorbic Acid (Vitamin C) and Its Role in the Common Cold, Tissue Healing, and Iron Metabolism. Cureus. 2023;15(11):e49308 https://doi.org/10.7759/cureus.49308.
https://doi.org/10.7759/cureus.49308 |
| 259 | Anderson GJ, Vulpe CD. Mammalian iron transport. Cell Mol Life Sci. 2009;66(20):3241-3261 https://doi.org/10.1007/s00018-009-0051-1.
https://doi.org/10.1007/s00018-009-0051-1 |
| 260 | Cook JD, Monsen ER. Vitamin C, the common cold, and iron absorption. Am J Clin Nutr. 1977;30(2):235-241 https://doi.org/10.1093/ajcn/30.2.235.
https://doi.org/10.1093/ajcn/30.2.235 |
| 261 | Fleming DJ, Jacques PF, Dallal GE, Tucker KL, Wilson PW, Wood RJ. Dietary determinants of iron stores in a free-living elderly population: The Framingham Heart Study. Am J Clin Nutr. 1998;67(4):722-733 https://doi.org/10.1093/ajcn/67.4.722.
https://doi.org/10.1093/ajcn/67.4.722 |
| 262 | Harrison-Findik DD. Role of alcohol in the regulation of iron metabolism. World J Gastroenterol. 2007;13(37):4925-4930 https://doi.org/10.3748/wjg.v13.i37.4925.
https://doi.org/10.3748/wjg.v13.i37.4925 |
| 263 | Butts M, Sundaram VL, Murughiyan U, Borthakur A, Singh S. The Influence of Alcohol Consumption on Intestinal Nutrient Absorption: A Comprehensive Review. Nutrients. 2023;15(7):1571 https://doi.org/10.3390/nu15071571.
https://doi.org/10.3390/nu15071571 |
| 264 | Reddy NR, Sathe SK, Salunkhe DK. Phytates in legumes and cereals. Adv Food Res. 1982;28:1-92 https://doi.org/10.1016/s0065-2628(08)60110-x.
https://doi.org/10.1016/S0065-2628(08)60110-X |
| 265 | Gupta RK, Gangoliya SS, Singh NK. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol. 2015;52(2):676-684 https://doi.org/10.1007/s13197-013-0978-y.
https://doi.org/10.1007/s13197-013-0978-y |
| 266 | Schlemmer U, Frølich W, Prieto RM, Grases F. Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res. 2009;53 Suppl 2:S330-S375 https://doi.org/10.1002/mnfr.200900099.
https://doi.org/10.1002/mnfr.200900099 |
| 267 | Zhang HW, Bai XL. Optimization of extraction conditions for phytic acid from rice bran using response surface methodology and its antioxidant effects. J Food Sci Technol. 2014;51(2):371-376 https://doi.org/10.1007/s13197-011-0521-y.
https://doi.org/10.1007/s13197-011-0521-y |
| 268 | Reddy MB, Hurrell RF, Juillerat MA, Cook JD. The influence of different protein sources on phytate inhibition of nonheme-iron absorption in humans. Am J Clin Nutr. 1996;63(2):203-207 https://doi.org/10.1093/ajcn/63.2.203.
https://doi.org/10.1093/ajcn/63.2.203 |
| 269 | Lestienne I, Caporiccio B, Besançon P, Rochette I, Trèche S. Relative contribution of phytates, fibers, and tannins to low iron and zinc in vitro solubility in pearl millet (Pennisetum glaucum) flour and grain fractions. J Agric Food Chem. 2005;53(21):8342-8348 https://doi.org/10.1021/jf050741p.
https://doi.org/10.1021/jf050741p |
| 270 | Heshe GG, Haki GD, Woldegiorgis AZ, Gemede HF. Effect of conventional milling on the nutritional value and antioxidant capacity of wheat types common in Ethiopia and a recovery attempt with bran supplementation in bread. Food Sci Nutr. 2015;4(4):534-543 https://doi.org/10.1002/fsn3.315.
https://doi.org/10.1002/fsn3.315 |
| 271 | Hurrell RF, Reddy MB, Burri J, Cook JD. An evaluation of EDTA compounds for iron fortification of cereal-based foods. Br J Nutr. 2000;84(6):903-910.
https://doi.org/10.1017/S0007114500002531 |
| 272 | Hurrell RF, Reddy M, Cook JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr. 1999;81(4):289-295.
https://doi.org/10.1017/S0007114599000537 |
| 273 | Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91(5):1461S-1467S. https://doi.org/10.3945/ajcn.2010.28674F.
https://doi.org/10.3945/ajcn.2010.28674F |
| 274 | Brune M, Rossander L, Hallberg L. Iron absorption and phenolic compounds: importance of different phenolic structures. Eur J Clin Nutr. 1989;43(8):547-557.
|
| 275 | Delimont NM, Haub MD, Lindshield BL. The Impact of Tannin Consumption on Iron Bioavailability and Status: A Narrative Review. Curr Dev Nutr. 2017;1(2):1-12 https://doi.org/10.3945/cdn.116.000042.
https://doi.org/10.3945/cdn.116.000042 |
| 276 | Chasteen ND, Harrison PM. Mineralization in ferritin: an efficient means of iron storage. J Struct Biol. 1999;126(3):182-194 https://doi.org/10.1006/jsbi.1999.4118.
https://doi.org/10.1006/jsbi.1999.4118 |
| 277 | Bjørklund G, Aaseth J, Skalny AV, Suliburska J, Skalnaya MG, Nikonorov AA, Tinkov AA. Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency. J Trace Elem Med Biol. 2017;41:41-53 https://doi.org/10.1016/j.jtemb.2017.02.005.
https://doi.org/10.1016/j.jtemb.2017.02.005 |
| 278 | Knez M, Graham RD, Welch RM, Stangoulis JC. New perspectives on the regulation of iron absorption via cellular zinc concentrations in humans. Crit Rev Food Sci Nutr. 2017;57(10):2128-2143 https://doi.org/10.1080/10408398.2015.1050483.
https://doi.org/10.1080/10408398.2015.1050483 |
| 279 | Pizarro F, Olivares M, Valenzuela C, Brito A, Weinborn V, Flores S, Arredondo M. The effect of proteins from animal source foods on heme iron bioavailability in humans. Food Chem. 2016;196:733-738 https://doi.org/10.1016/j.foodchem.2015.10.012.
https://doi.org/10.1016/j.foodchem.2015.10.012 |
| 280 | Zappacosta B, Persichilli S, Iacoviello L, Di Castelnuovo A, Graziano M, Gervasoni J, Leoncini E, Cimino G, Mastroiacovo P. Folate, vitamin B12 and homocysteine status in an Italian blood donor population. Nutr Metab Cardiovasc Dis. 2013;23(5):473-480 https://doi.org/10.1016/j.numecd.2011.10.001.
https://doi.org/10.1016/j.numecd.2011.10.001 |
| 281 | Krzywański J, Mikulski T, Pokrywka A, Młyńczak M, Krysztofiak H, Frączek B, Ziemba A. Vitamin B12 Status and Optimal Range for Hemoglobin Formation in Elite Athletes. Nutrients. 2020;12(4):1038 https://doi.org/10.3390/nu12041038.
https://doi.org/10.3390/nu12041038 |
| 282 | Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. 2004;24:105-131 https://doi.org/10.1146/annurev.nutr.24.012003.132306.
https://doi.org/10.1146/annurev.nutr.24.012003.132306 |
| 283 | Allen LH, Miller JW, de Groot L, Rosenberg IH, Smith AD, Refsum H, Raiten DJ. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J Nutr. 2018;148(suppl_4):1995S-2027S. https://doi.org/10.1093/jn/nxy201.
https://doi.org/10.1093/jn/nxy201 |
| 284 | Watanabe F, Bito T. Vitamin B12 sources and microbial interaction. Exp Biol Med (Maywood). 2018;243(2):148-158 https://doi.org/10.1177/1535370217746612.
https://doi.org/10.1177/1535370217746612 |
| 285 | Watanabe F. Vitamin B12 sources and bioavailability. Exp Biol Med (Maywood). 2007;232(10):1266-1274 https://doi.org/10.3181/0703-MR-67.
https://doi.org/10.3181/0703-MR-67 |
| 286 | Obeid R, Heil SG, Verhoeven MMA, van den Heuvel EGHM, de Groot LCPGM, Eussen SJPM. Vitamin B12 Intake From Animal Foods, Biomarkers, and Health Aspects. Front Nutr. 2019;6:93 https://doi.org/10.3389/fnut.2019.00093.
https://doi.org/10.3389/fnut.2019.00093 |
| 287 | Gille D, Schmid A. Vitamin B12 in meat and dairy products. Nutr Rev. 2015;73(2):106-115 https://doi.org/10.1093/nutrit/nuu011.
https://doi.org/10.1093/nutrit/nuu011 |
| 288 | Doscherholmen A, McMahon J, Ripley D. Vitamin B12 absorption from eggs. Proc Soc Exp Biol Med. 1975;149(4):987-990 https://doi.org/10.3181/00379727-149-38940.
https://doi.org/10.3181/00379727-149-38940 |
| 289 | Watanabe F, Schwarz J, Takenaka S, Miyamoto E, Ohishi N, Nelle E, Hochstrasser R, Yabuta Y. Characterization of vitamin B₁₂ compounds in the wild edible mushrooms black trumpet (Craterellus cornucopioides) and golden chanterelle (Cantharellus cibarius). J Nutr Sci Vitaminol (Tokyo). 2012;58(6):438-441 https://doi.org/10.3177/jnsv.58.438.
https://doi.org/10.3177/jnsv.58.438 |
| 290 | Carmel R. Folic acid. In: Shils M, Shike M, Ross A, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins 2005:470-81.
|
| 291 | Allen LH. Vitamin B-12 Adv Nutr. 2012;3(1):54-55 https://doi.org/10.3945/an.111.001370.
https://doi.org/10.3945/an.111.001370 |
| 292 | Froese DS, Fowler B, Baumgartner MR. Vitamin B12, folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J Inherit Metab Dis. 2019;42(4):673-685 https://doi.org/10.1002/jimd.12009.
https://doi.org/10.1002/jimd.12009 |
| 293 | Metz J. Cobalamin deficiency and the pathogenesis of nervous system disease. Annu Rev Nutr. 1992;12:59-79 https://doi.org/10.1146/annurev.nu.12.070192.000423.
https://doi.org/10.1146/annurev.nu.12.070192.000423 |
| 294 | Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020;12(1):236 https://doi.org/10.3390/nu12010236.
https://doi.org/10.3390/nu12010236 |
| 295 | Chen Y, Michalak M, Agellon LB. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J Biol Med. 2018;91(2):95-103.
|
| 296 | Tardy AL, Pouteau E, Marquez D, Yilmaz C, Scholey A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients. 2020;12(1):228 https://doi.org/10.3390/nu12010228.
https://doi.org/10.3390/nu12010228 |
| 297 | Huang Z, Liu Y, Qi G, Brand D, Zheng SG: Role of Vitamin A in the Immune System. J Clin Med. 2018;7(9):258 https://doi.org/10.3390/jcm7090258.
https://doi.org/10.3390/jcm7090258 |
| 298 | McGrane MM. Vitamin A regulation of gene expression: molecular mechanism of a prototype gene. J Nutr Biochem. 2007;18(8):497-508 https://doi.org/10.1016/j.jnutbio.2006.10.006.
https://doi.org/10.1016/j.jnutbio.2006.10.006 |
| 299 | Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res. 2013;54(7):1761-1775 https://doi.org/10.1194/jlr.R030833.
https://doi.org/10.1194/jlr.R030833 |
| 300 | Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001. 4, Vitamin A. Available from: https://www.ncbi.nlm.nih.gov/books/NBK222318/.
|
| 301 | Weber D, Grune T. The contribution of β-carotene to vitamin A supply of humans. Mol Nutr Food Res. 2012;56(2):251-258 https://doi.org/10.1002/mnfr.201100230.
https://doi.org/10.1002/mnfr.201100230 |
| 302 | Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutr J. 2003;2:7 https://doi.org/10.1186/1475-2891-2-7.
https://doi.org/10.1186/1475-2891-2-7 |
| 303 | Padayatty SJ, Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 2016;22(6):463-493 https://doi.org/10.1111/odi.12446.
https://doi.org/10.1111/odi.12446 |
| 304 | Li Y, Schellhorn HE: New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137(10):2171-2184 https://doi.org/10.1093/jn/137.10.2171.
https://doi.org/10.1093/jn/137.10.2171 |
| 305 | DePhillipo NN, Aman ZS, Kennedy MI, Begley JP, Moatshe G, LaPrade RF. Efficacy of Vitamin C Supplementation on Collagen Synthesis and Oxidative Stress After Musculoskeletal Injuries: A Systematic Review. Orthop J Sports Med. 2018;6(10):2325967118804544 https://doi.org/10.1177/2325967118804544.
https://doi.org/10.1177/2325967118804544 |
| 306 | Gregory JF 3rd: Ascorbic acid bioavailability in foods and supplements. Nutr Rev. 1993;51(10):301-303 https://doi.org/10.1111/j.1753-4887.1993.tb03059.x.
https://doi.org/10.1111/j.1753-4887.1993.tb03059.x |
| 307 | Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F. The role of vitamin e in human health and some diseases. Sultan Qaboos Univ Med J. 2014;14(2):e157-e165.
|
| 308 | Burton GW, Joyce A, Ingold KU. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes?. Arch Biochem Biophys. 1983;221(1):281-290 https://doi.org/10.1016/0003-9861(83)90145-5.
https://doi.org/10.1016/0003-9861(83)90145-5 |
| 309 | Tran K, Wong JT, Lee E, Chan AC, Choy PC. Vitamin E potentiates arachidonate release and phospholipase A2 activity in rat heart myoblastic cells. Biochem J. 1996;319 (Pt 2):385-391 https://doi.org/10.1042/bj3190385.
https://doi.org/10.1042/bj3190385 |
| 310 | Davi G, Alessandrini P, Mezzetti A, Minotti G, Bucciarelli T, Costantini F, Cipollone F, Bon GB, Ciabattoni G, Patrono C. In vivo formation of 8-Epi-prostaglandin F2 alpha is increased in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17(11):3230-3235 https://doi.org/10.1161/01.atv.17.11.3230.
https://doi.org/10.1161/01.ATV.17.11.3230 |
| 311 | Davì G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, Capani F, Patrono C In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99(2):224-229 https://doi.org/10.1161/01.cir.99.2.224.
https://doi.org/10.1161/01.CIR.99.2.224 |
| 312 | Hayden SJ, Albert TJ, Watkins TR, Swenson ER. Anemia in critical illness: insights into etiology, consequences, and management. Am J Respir Crit Care Med. 2012;185(10):1049-1057 https://doi.org/10.1164/rccm.201110-1915CI.
https://doi.org/10.1164/rccm.201110-1915CI |
| 313 | Wessels I, Maywald M, Rink L. Zinc as a Gatekeeper of Immune Function. Nutrients. 2017;9(12):1286 https://doi.org/10.3390/nu9121286.
https://doi.org/10.3390/nu9121286 |
| 314 | Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68(2 Suppl):447S-463S. https://doi.org/10.1093/ajcn/68.2.447S.
https://doi.org/10.1093/ajcn/68.2.447S |
| 315 | Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14(5-6):353-357 https://doi.org/10.2119/2008-00033.Prasad.
https://doi.org/10.2119/2008-00033.Prasad |
| 316 | Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25(1):11-24 https://doi.org/10.1007/s10787-017-0309-4.
https://doi.org/10.1007/s10787-017-0309-4 |
| 317 | An Y, Li S, Huang X, Chen X, Shan H, Zhang M. The Role of Copper Homeostasis in Brain Disease. Int J Mol Sci. 2022;23(22):13850 https://doi.org/10.3390/ijms232213850.
https://doi.org/10.3390/ijms232213850 |
| 318 | Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4(3):176-185 https://doi.org/10.1038/nchembio.72.
https://doi.org/10.1038/nchembio.72 |
| 319 | Borkow G. Using Copper to Improve the Well-Being of the Skin. Curr Chem Biol. 2014;8(2):89-102 https://doi.org/10.2174/2212796809666150227223857.
https://doi.org/10.2174/2212796809666150227223857 |
| 320 | Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101(3):294-301 https://doi.org/10.1016/S0002-8223(01)00078-5.
https://doi.org/10.1016/S0002-8223(01)00078-5 |
| 321 | Pierson H, Yang H, Lutsenko S. Copper Transport and Disease: What Can We Learn from Organoids?. Annu Rev Nutr. 2019;39:75-94 https://doi.org/10.1146/annurev-nutr-082018-124242.
https://doi.org/10.1146/annurev-nutr-082018-124242 |
| 322 | Rahman MM, Karki S, Hayen A. A methods review of the "healthy donor effect" in studies of long-term health outcomes in blood donors. Transfusion. 2022;62(3):698-712 https://doi.org/10.1111/trf.16791.
https://doi.org/10.1111/trf.16791 |
| 323 | Atsma F, de Vegt F. The healthy donor effect: a matter of selection bias and confounding. Transfusion. 2011;51(9):1883-1885 https://doi.org/10.1111/j.1537-2995.2011.03270.x.
https://doi.org/10.1111/j.1537-2995.2011.03270.x |
| 324 | France CR, Ditto B, Wissel ME, France JL, Dickert T, Rader A, Sinclair K, McGlone S, Trost Z, Matson E. Predonation hydration and applied muscle tension combine to reduce presyncopal reactions to blood donation. Transfusion. 2010;50(6):1257-1264 https://doi.org/10.1111/j.1537-2995.2009.02574.x.
https://doi.org/10.1111/j.1537-2995.2009.02574.x |
| 325 | Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463(1):121-137 https://doi.org/10.1007/s00424-011-1044-0.
https://doi.org/10.1007/s00424-011-1044-0 |
| 326 | Vyazovskiy VV. Sleep, recovery, and metaregulation: explaining the benefits of sleep. Nat Sci Sleep. 2015;7:171-184 https://doi.org/10.2147/NSS.S54036.
https://doi.org/10.2147/NSS.S54036 |
| 327 | Mousavi SA, Hermundstad B, Saether PC, Nybruket MJ, Knutsen TR, Llohn AH. Health Behavior and Lifestyle Trends among Platelet Donors: Results from a Questionnaire-Based Survey in Norway. Biomed Res Int. 2021;2021:8891885 https://doi.org/10.1155/2021/8891885.
https://doi.org/10.1155/2021/8891885 |
| 328 | Montero D, Breenfeldt-Andersen A, Oberholzer L, Haider T, Goetze JP, Meinild-Lundby AK, Lundby C. Erythropoiesis with endurance training: dynamics and mechanisms. Am J Physiol Regul Integr Comp Physiol. 2017;312(6):R894-R902 https://doi.org/10.1152/ajpregu.00012.2017.
https://doi.org/10.1152/ajpregu.00012.2017 |
| 329 | Montero D, Lundby C. Regulation of Red Blood Cell Volume with Exercise Training. Compr Physiol. 2018;9(1):149-164 https://doi.org/10.1002/cphy.c180004.
https://doi.org/10.1002/cphy.c180004 |
| 330 | Lyle RM, Weaver CM, Sedlock DA, Rajaram S, Martin B, Melby CL. Iron status in exercising women: the effect of oral iron therapy vs increased consumption of muscle foods. Am J Clin Nutr. 1992;56(6):1049-1055 https://doi.org/10.1093/ajcn/56.6.1049.
https://doi.org/10.1093/ajcn/56.6.1049 |
| 331 | Shoemaker JK, Green HJ, Coates J, Ali M, Grant S. Failure of prolonged exercise training to increase red cell mass in humans. Am J Physiol. 1996;270(1 Pt 2):H121-H126 https://doi.org/10.1152/ajpheart.1996.270.1.H121.
https://doi.org/10.1152/ajpheart.1996.270.1.H121 |
| 332 | Ziegler AK, Grand J, Stangerup I, Nielsen HJ, Dela F, Magnussen K, Helge JW. Time course for the recovery of physical performance, blood hemoglobin, and ferritin content after blood donation. Transfusion. 2015;55(4):898-905 https://doi.org/10.1111/trf.12926.
https://doi.org/10.1111/trf.12926 |
| 333 | Judd TB, Cornish SM, Barss TS, Oroz I, Chilibeck PD. Time course for recovery of peak aerobic power after blood donation. J Strength Cond Res. 2011;25(11):3035-3038 https://doi.org/10.1519/JSC.0b013e3182132df7.
https://doi.org/10.1519/JSC.0b013e3182132df7 |
| 334 | Eckstrom E, Neukam S, Kalin L, Wright J. Physical Activity and Healthy Aging. Clin Geriatr Med. 2020;36(4):671-683 https://doi.org/10.1016/j.cger.2020.06.009.
https://doi.org/10.1016/j.cger.2020.06.009 |
| 335 | Swift DL, McGee JE, Earnest CP, Carlisle E, Nygard M, Johannsen NM. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog Cardiovasc Dis. 2018;61(2):206-213 https://doi.org/10.1016/j.pcad.2018.07.014.
https://doi.org/10.1016/j.pcad.2018.07.014 |
| 336 | Halon-Golabek M, Borkowska A, Herman-Antosiewicz A, Antosiewicz J. Iron Metabolism of the Skeletal Muscle and Neurodegeneration. Front Neurosci. 2019;13:165 https://doi.org/10.3389/fnins.2019.00165.
https://doi.org/10.3389/fnins.2019.00165 |
| 337 | Mairbäurl H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front Physiol. 2013;4:332 https://doi.org/10.3389/fphys.2013.00332.
https://doi.org/10.3389/fphys.2013.00332 |
| 338 | Solberg A, Reikvam H. Iron Status and Physical Performance in Athletes. Life (Basel). 2023;13(10):2007 https://doi.org/10.3390/life13102007.
https://doi.org/10.3390/life13102007 |
| 339 | Skarpańska-Stejnborn A, Basta P, Trzeciak J, Szcześniak-Pilaczyńska Ł. Effect of intense physical exercise on hepcidin levels and selected parameters of iron metabolism in rowing athletes. Eur J Appl Physiol. 2015;115(2):345-351 https://doi.org/10.1007/s00421-014-3018-3.
https://doi.org/10.1007/s00421-014-3018-3 |
| 340 | Peeling P. Exercise as a mediator of hepcidin activity in athletes. Eur J Appl Physiol. 2010;110(5):877-883 https://doi.org/10.1007/s00421-010-1594-4.
https://doi.org/10.1007/s00421-010-1594-4 |
| 341 | Crouter SE, DellaValle DM, Haas JD. Relationship between physical activity, physical performance, and iron status in adult women. Appl Physiol Nutr Metab. 2012;37(4):697-705 https://doi.org/10.1139/h2012-044.
https://doi.org/10.1139/h2012-044 |
| 342 | Lukaski HC. Vitamin and mineral status: effects on physical performance. Nutrition. 2004;20(7-8):632-644 https://doi.org/10.1016/j.nut.2004.04.001.
https://doi.org/10.1016/j.nut.2004.04.001 |
| 343 | Buratti P, Gammella E, Rybinska I, Cairo G, Recalcati S. Recent Advances in Iron Metabolism: Relevance for Health, Exercise, and Performance. Med Sci Sports Exerc. 2015;47(8):1596-1604 https://doi.org/10.1249/MSS.0000000000000593.
https://doi.org/10.1249/MSS.0000000000000593 |
| 344 | Qiu Y, Fernández-García B, Lehmann HI, Li G, Kroemer G, López-Otín C, Xiao J. Exercise sustains the hallmarks of health. J Sport Health Sci. 2023;12(1):8-35 https://doi.org/10.1016/j.jshs.2022.10.003.
https://doi.org/10.1016/j.jshs.2022.10.003 |
| 345 | Chatterjee A, Prinz A, Gerdes M, Martinez S. Digital Interventions on Healthy Lifestyle Management: Systematic Review. J Med Internet Res. 2021;23(11):e26931 https://doi.org/10.2196/26931.
https://doi.org/10.2196/26931 |
| 346 | Touvier M, da Costa Louzada ML, Mozaffarian D, Baker P, Juul F, Srour B. Ultra-processed foods and cardiometabolic health: public health policies to reduce consumption cannot wait. BMJ. 2023;383:e075294 https://doi.org/10.1136/bmj-2023-075294.
https://doi.org/10.1136/bmj-2023-075294 |
| 347 | Nguyen DD, Devita DA, Hirschler NV, Murphy EL. Blood donor satisfaction and intention of future donation. Transfusion. 2008;48(4):742-748 https://doi.org/10.1111/j.1537-2995.2007.01600.x.
https://doi.org/10.1111/j.1537-2995.2007.01600.x |
| 348 | Gautam S, Jain A, Chaudhary J, Gautam M, Gaur M, Grover S. Concept of mental health and mental well-being, it's determinants and coping strategies. Indian J Psychiatry. 2024;66(Suppl 2):S231-S244 https://doi.org/10.4103/indianjpsychiatry.indianjpsychiatry_707_23.
https://doi.org/10.4103/indianjpsychiatry.indianjpsychiatry_707_23 |
| 349 | Almeida RG, Martinez EZ, Mazzo A, Trevizan MA, Mendes IA. Spirituality and post-graduate students' attitudes towards blood donation. Nurs Ethics. 2013;20(4):392-400 https://doi.org/10.1177/0969733012465999.
https://doi.org/10.1177/0969733012465999 |
| 350 | Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients. 2016;8(1):56 https://doi.org/10.3390/nu8010056.
https://doi.org/10.3390/nu8010056 |
| 351 | O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32-48 https://doi.org/10.1016/j.bbr.2014.07.027.
https://doi.org/10.1016/j.bbr.2014.07.027 |
| 352 | Yim J: Therapeutic Benefits of Laughter in Mental Health: A Theoretical Review. Tohoku J Exp Med. 2016;239(3):243-249 https://doi.org/10.1620/tjem.239.243.
https://doi.org/10.1620/tjem.239.243 |
| 353 | Bellitti P, Valeriano R, Gasperi M, Sodini L, Barletta D. Cortisol and heart rate changes in first- and fourth-time donors. Vox Sang. 1994;67(1):42-45 https://doi.org/10.1111/j.1423-0410.1994.tb05036.x.
https://doi.org/10.1111/j.1423-0410.1994.tb05036.x |
| 354 | Hinrichs A, Picker SM, Schneider A, Lefering R, Neugebauer EA, Gathof BS. Effect of blood donation on well-being of blood donors. Transfus Med. 2008;18(1):40-48 https://doi.org/10.1111/j.1365-3148.2007.00805.x.
https://doi.org/10.1111/j.1365-3148.2007.00805.x |
| 355 | Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163-171 https://doi.org/10.1016/j.psyneuen.2008.10.026.
https://doi.org/10.1016/j.psyneuen.2008.10.026 |
| 356 | Shamay-Tsoory SG, Abu-Akel A. The Social Salience Hypothesis of Oxytocin. Biol Psychiatry. 2016;79(3):194-202 https://doi.org/10.1016/j.biopsych.2015.07.020.
https://doi.org/10.1016/j.biopsych.2015.07.020 |
| 357 | Ebert A, Brüne M. Oxytocin and Social Cognition. Curr Top Behav Neurosci. 2018;35:375-388 https://doi.org/10.1007/7854_2017_21.
https://doi.org/10.1007/7854_2017_21 |
| 358 | Jukić I, Kovač D, Vuletić Čugalj D. Oxytocin, empathy, altruism and charitable giving: Experimental evidence from blood donations, IWH Discussion Papers, No. 4/2023, Halle Institute for Economic Research (IWH), Halle (Saale), https://nbn-resolving.de/urn:nbn:de:gbv:3:2-937492.
|
| 359 | Lu HC, Mackie K. Review of the Endocannabinoid System. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6(6):607-615 https://doi.org/10.1016/j.bpsc.2020.07.016.
https://doi.org/10.1016/j.bpsc.2020.07.016 |
| 360 | Saleh-Ghadimi S, Kheirouri S, Maleki V, Jafari-Vayghan H, Alizadeh M. Endocannabinoid system and cardiometabolic risk factors: A comprehensive systematic review insight into the mechanistic effects of omega-3 fatty acids. Life Sci. 2020;250:117556 https://doi.org/10.1016/j.lfs.2020.117556.
https://doi.org/10.1016/j.lfs.2020.117556 |
| 361 | Hulsken S, Märtin A, Mohajeri MH, Homberg JR. Food-derived serotonergic modulators: effects on mood and cognition. Nutr Res Rev. 2013;26(2):223-234 https://doi.org/10.1017/S0954422413000164.
https://doi.org/10.1017/S0954422413000164 |
| 362 | Strasser B, Gostner JM, Fuchs D. Mood, food, and cognition: role of tryptophan and serotonin. Curr Opin Clin Nutr Metab Care. 2016;19(1):55-61 https://doi.org/10.1097/MCO.0000000000000237.
https://doi.org/10.1097/MCO.0000000000000237 |
| 363 | Naidoo U. Eat to Beat Stress. Am J Lifestyle Med. 2020;15(1):39-42 https://doi.org/10.1177/1559827620973936.
https://doi.org/10.1177/1559827620973936 |
| 364 | Alsaffar AA. Sustainable diets: The interaction between food industry, nutrition, health and the environment. Food Sci Technol Int. 2016;22(2):102-111 https://doi.org/10.1177/1082013215572029.
https://doi.org/10.1177/1082013215572029 |