Original Article - DOI:10.33594/000000874
Accepted 26 January 2026 - Published online
7 February 2026
Relationship Between Left Ventricular Remodeling Assessed by Strain Echocardiography and Conventional Echocardiography Before and After Cardiac Resynchronization Therapy in Severe Heart Failure Patients
bHospital of Vietnam National University, Vietnam National University (Hanoi), Vietnam
Keywords
Abstract
Background/Aims:
To assess the relationship between left ventricular remodeling as evaluated by strain echocardiography and conventional echocardiography before and after cardiac resynchronization therapy (CRT) in patients with severe heart failure patients.Methods:
A cross-sectional descriptive study with follow-up comparison before and after. The study was conducted on 33 heart failure patients indicated for CRT at the Vietnam National Heart Institute, Bach Mai Hospital from late 2015 to December 2021.Results:
The study showed some correlations and changes in left ventricular remodeling assessed by strain echocardiography and conventional echocardiography before and after CRT. Left ventricular end-systolic volume and end-diastolic volume significantly decreased after 3 months of follow-up (p < 0.001) and had a strong inverse correlation with QRS recovery in study patients (r = 0.65). A positive correlation was observed between changes in left atrial volume index (LAVI) and 4B strain (r = 0.96, p < 0.05). After 3 months of CRT implantation, there was a positive correlation between the isovolumic relaxation time (IVRT) and left atrial volume (LAVI) (p < 0.05) and between the left atrial volume and E/e' obtained from tissue Doppler (r = 0.53, p < 0.05). However, there was no correlation observed between E/e' and total EF (r = -0.36, p > 0.05), between 2B strain and total EF (r = -0.36, p > 0.05) and between 2B strain and left atrial volume LAVI (r = 0.36, p > 0.05).Conclusion:
Strain echocardiography is an important tool that cannot be overlooked in diagnosing, assessing risk factors, and monitoring heart failure. After 3 months of CRT implantation, there was significant improvement in left ventricular volume and ejection fraction (EF). This indicates better treatment effectiveness.Introduction
Globally, the prevalence of heart failure is 1-2% [1], and it is most common in the elderly. In Vietnam, the incidence of heart failure is also high [2], and following global trends, Vietnam is also performing Cardiac Resynchronization Therapy (CRT) for patients with severe heart failure who meet the indications [3].
The emergence and development of CRT since the 1990s have opened a new direction in heart failure treatment and have raised many new issues related to the pathogenesis of heart failure, especially in the context of cardiac remodeling and myocardial dyssynchrony (MDB) [3].
Echocardiography helps assess ejection fraction (EF), and is an important tool for evaluating cardiac function, identifying structural abnormalities, helping to determine the cause of heart failure or other risk factors, helping to monitor early treatment, assessing heart function improvement and related factors. As people age, natural aging processes can cause changes in heart structure such as thickening heart walls, enlarged chambers, and reduced contractility. Simultaneously, an aging population also faces an increased risk of cardiovascular diseases like coronary artery disease, hypertension, and valvular heart disease. However, studies on the relationship and changes in left ventricular function have been conducted worldwide, but an in-depth assessment of heart failure patients indicated for CRT has not been extensively investigated. Therefore, we conducted this study to understand the relationship between left ventricular remodeling assessed by strain echocardiography before and after cardiac resynchronization therapy compared to conventional echocardiography in heart failure patients indicated for CRT.
Materials and Methods
Research Design
The study was conducted as a cross-sectional descriptive study with follow-up comparison before and after.
Study Subjects: The study subjects included patients with severe heart failure, with an EF <
35%, who
were undergoing optimal medical treatment for heart failure in NYHA III - IV, and who were indicated for
cardiac
resynchronization therapy based on echocardiographic results, electrocardiogram, biochemical results, and
clinical symptoms when implanted with cardiac resynchronization devices at Bach Mai Hospital Heart
Institute.
Patients receiving cardiac resynchronization devices were selected based on ACC/AHA 2008 guidelines [4].
Time and location research
The study was conducted at the Vietnam National Heart Institute, Bach Mai Hospital, from late 2015 to
December
2021.
Sampling Method
Convenience sampling. A total of 33 patients who met the inclusion criteria and agreed to participate in
the
study were selected. These were all patients who met the criteria and followed all steps in the research
process.
Research Progress
Clinical Examination: All patients underwent a general examination to assess heart failure status
NYHA
III – IV (NYHA Class I: Cardiac disease, but no symptoms and no limitaion in ordinary physical activity;
NYHA
Class II: Mild symptoms and slight limitations during ordinary activity; NYHA Class III: Significant
limitations
in activity due to symptoms, comfortalbe only at rest.; NYHA Class IV: Servere limitations, symptoms even
while
at rest).
The follow-up period after implantation was 6 months. Patients were treated according to ACC/AHA 2008
guidelines
for at least 6 months. [4]. Patients received priority treatment for various types of medications such as
ACE
inhibitors, ARBs, Beta-blockers, and Digoxin.
Biochemical Tests
Some main biochemical parameters were collected, including: Pro BNP, Urea, Creatinine, SGOT, SGPT.
Imaging: Doppler ultrasound, tissue Doppler ultrasound. The classify heart failure (HF) based on
Left
ventricular Ejection Fraction (LVEF): HF with Reduced Ejection Fraction (HfrEF) is EF ≤ 40%; HF with Midly
Reduced Ejection Fraction (HfmrEF) 41-49%; FF with Preserved Ejection Fraction (HfpEF) ≤ 50%.
Research procedure (Research diagram)
Select patients with severe heart failure (NYHA III – IV), EF < 35%, after optimal medical treatment,
sinus
rhythm was restored. Perform Doppler echocardiography beforehand. Patients with indications for pacemaker
implantation will have a CRT implanted. Each patient will undergo echocardiography 4 times: Before
pacemaker
implantation, immediately after pacemaker implantation, after 1 month, and after 3 months.
Cardiac Doppler Ultrasound Method
The Cardiac Doppler ultrasound was performed on all selected patients participating in the study using a
JIS6 ultrasound machine at the cardiac ultrasound room of the Vietnam National Heart Institute. The
measured results were assessed by a group including cardiac ultrasound specialists at the cardiac
ultrasound
department of the Vietnam National Heart Institute. Ultrasound parameters were measured and
calculated
according to the guidelines of the American Society of Echocardiography (2008) on an ultrasound machine
with
Tissue Doppler and myocardial tissue tagging functions, using the JIS6 ultrasound
machine.4
The ultrasound parameters were examined in the following sequence (Fig. 1).
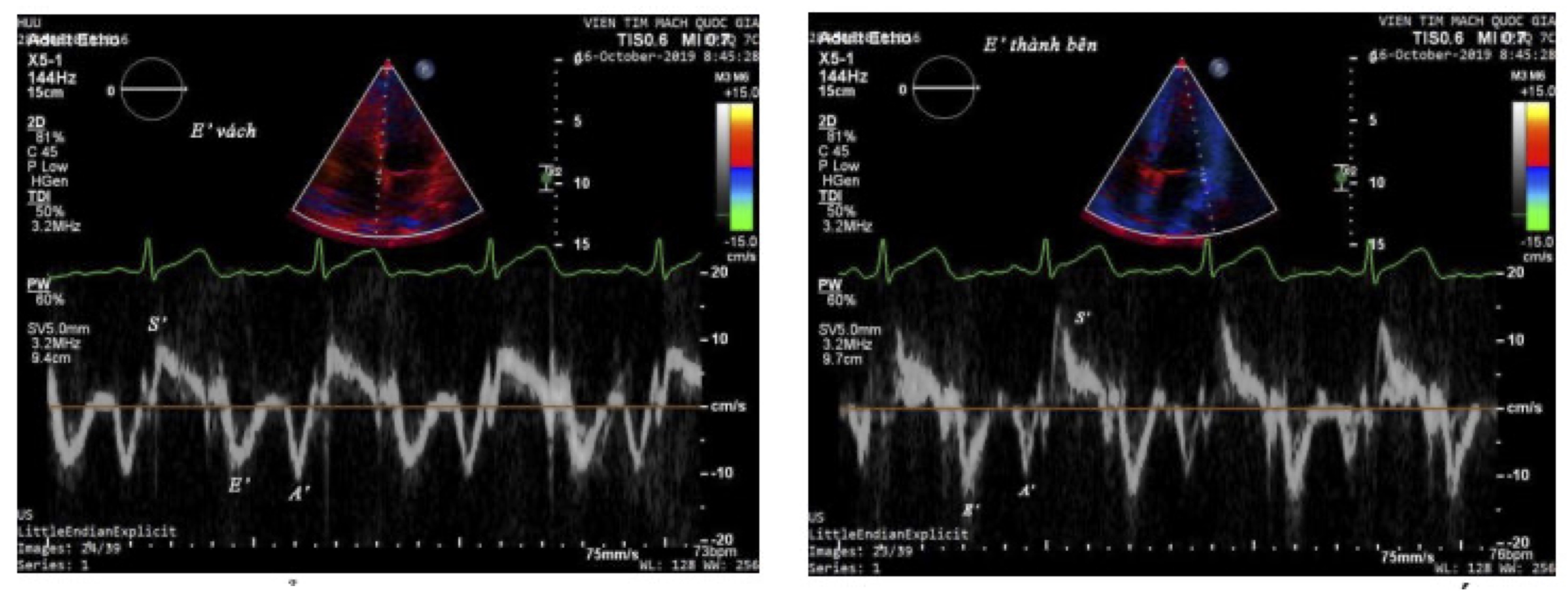
Fig. 1: Doppler ultrasound image of myocardial tissue
M-Mode Echocardiography
Measured from the parasternal long axis view, the following parameters were measured: Dd: left ventricular
end-diastolic diameter; Ds: left ventricular end-systolic diameter; %D: fractional shortening; Vd: left
ventricular end-diastolic volume; Vs: left ventricular end-systolic volume; Aortic diameter, left atrial
diameter, and right ventricular long-axis diameter; EF (Teichholz): left ventricular ejection fraction.
2D Echocardiography
Measured from the apical four-chamber and two-chamber views. Left ventricular volume and ejection fraction
were
calculated using the Biplane Simpson's method (four-chamber and two-chamber views). Left ventricular
end-systolic volume and end-diastolic volume were measured. The parameters for left ventricular
end-diastolic volume (Vd) and end-systolic volume (Vs) were averaged from the two views (Biplane). The
average
left atrial volume index (LAVi) was calculated from both the apical four-chamber and two-chamber views.
Pulsed Wave Doppler Echocardiography
Measured from the apical four-chamber and five-chamber views. In the apical four-chamber view, the Pulsed
Wave
Doppler sample volume was placed at the free edge of the anterior mitral leaflet, measuring the maximum
velocity
of E wave, A wave, and E wave deceleration time (DT). In the apical five-chamber view, the Pulsed Wave
Doppler
sample volume was placed between the left ventricular outflow tract and the free edge of the anterior
mitral
leaflet, measuring the isovolumic relaxation time (IVRT).
Continuous Wave and Color Doppler Echocardiography
Measured from the apical four-chamber and two-chamber views.
Tissue Doppler Echocardiography
Measured from the apical four-chamber view with the Tissue software available on the ultrasound machine.
The
Doppler sample volume was placed at the basal interventricular septum using the TDI software, measuring
the
velocity of e', a', and s' waves. The Doppler sample volume was placed at the basal lateral wall of the
left
ventricle using the TDI software, measuring the velocity of e', a', and s' waves.
Speckle Tracking Echocardiography (Myocardial Tissue Tagging)
Measured from the apical long-axis, two-chamber, and four-chamber views, using the Speck tracking software
to
measure online and calculate the myocardial strain parameter; This includes Longitudinal Strain,
Four-Chamber Strain, Two-Chamber Strain, and Global Longitudinal Strain (GLS) for all cardiac chambers.
Whether
strain analysis was performed by a team of echocardiography experts at the Vietnam National Heart
Institute, and
the final analysin result and agreed upon by the team and the expert leader.
Doppler Ultrasound to Evaluate Cardiac Resynchronization Therapy (CRT) Response
Patients underwent Doppler echocardiography with all the same parameters as measured before CRT device
implantation. Each patient had echocardiography performed immediately after CRT implantation at 1
month,
and at 3 months post-implantation, and these parameters were monitored. The EF value presentation
was
clarified (Change in EF value relative to baseline).
Data Collection Method
All data was collected according to a pre-designed patient follow-up form. Collected data included
demographic
characteristics, clinical symptoms, and echocardiographic parameters of patients (echocardiography
collected
according to hospital results) upon admission and until the 3rd month after cardiac resynchronization
device
implantation.
Data processing and statistical analysis method
Data was processed using SPSS 17.0 (SPSS Inc South Wacker Drive, Chicago, IL) and Stata 12.0 to identify
significant values and find statistically significant parameters. The results were presented using tables
or
appropriate graphs; for continuous variables, results were presented as mean ± standard deviation, for
categorical variables, results were presented as percentages. T-test (for 2 variables), ANOVA test (for
more
than 2 variables) were used to compare continuous variables; Pearson correlation test was used to examine
the
relationship between categorical variables, Fisher's exact test was used for small sample size categorical
variables. Odds ratios (OR) were used to analyze factors influencing the results of pacemaker implantation
in a
2x2 table. Statistically significant differences were defined as p < 0.05 [5].
Research Ethics
The study was only conducted with the consent of participating patients. Patients participating in the
study
were treated according to the professional regulations of the Vietnam Ministry of Health, as well as those
of
the Vietnam National Heart Institute, Bach Mai Hospital. All patient information was kept absolutely
confidential, and the results were used solely for research purposes.
Results
General Characteristics of the Study Population
The study included 33 patients with severe heart failure indicated for CRT. The mean age of the patients
was
57.8 years; patients aged ≥60 years accounted for 57.6% (n = 19), while patients <60 years accounted
for
42.4% (n = 14). The male-to-female ratio was 81.8% (n = 27) to 18.2% (n = 6).
Changes in Conventional Echocardiographic Parameters After CRT
After 3 months of CRT implantation, left ventricular end-systolic volume (LVESV) and end-diastolic volume
(LVEDV) significantly decreased compared with baseline (both p < 0.001). Left ventricular ejection
fraction
(EF) also showed a significant improvement after CRT implantation (Table 1).
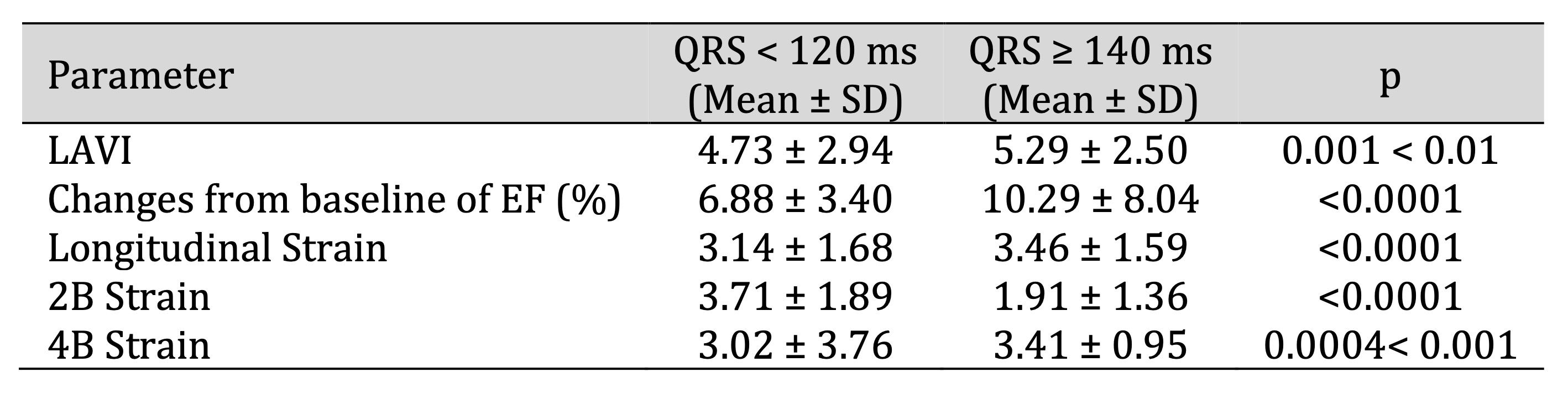
Table 1: Relationship of Positive change in echocardiographic parameters according to QRS duration at baseline and after 3 months of CRT implantation. Comments: Left ventricular volume and EF significantly improved after CRT implantation after 3 months, with p < 0.001. There was a strong inverse correlation with QRS recovery on the electrocardiogram of patients in the study group, with a correlation coefficient of 0.65
Relationship Between QRS Duration and Left Ventricular Remodeling
After 3 months of CRT implantation, QRS duration significantly decreased compared with baseline (p <
0.001).
QRS recovery showed a strong inverse correlation with left ventricular volumes, with a correlation
coefficient
of r = 0.65 (Table 1).
Changes in EF differed according to QRS duration. In patients with QRS between 120 and 140 ms, the mean
change
in EF was 6.88 ± 3.40%, whereas in patients with QRS ≥140 ms, EF increased by 10.29 ± 8.04% (Table 1).
Significant changes in myocardial strain parameters were also observed according to QRS recovery after
CRT
implantation (Table 1).
Relationship Between Left Atrial Volume and Myocardial Strain
A significant positive correlation was observed between changes in left atrial volume index (LAVI) and
four-chamber (4B) myocardial strain in patients with QRS ≥140 ms (r = 0.96, p < 0.05) (Table 2).
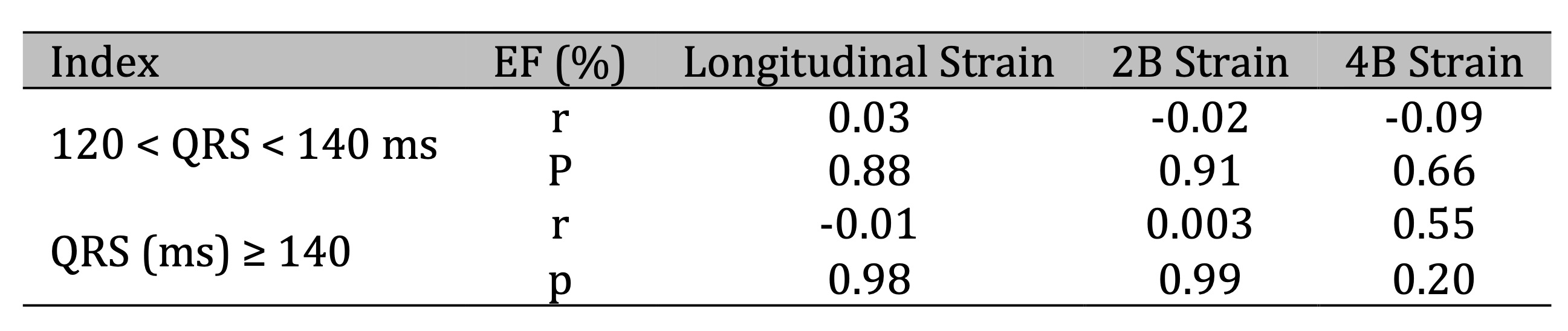
Table 2: Relationship between positive changes in left atrial volume (LAVI) and myocardial strain according to QRS duration before and after CRT implantation. Comments: There was a significant positive correlation between the positive change in left atrial volume (LAVI) and myocardial 4B strain in the QRS (ms) ≥ 140 group (r = 0.96, p < 0.05)
Diastolic Function Parameters After CRT
At 3 months after CRT implantation, a significant inverse correlation was observed between E/e′ ratio
and
total
EF (r = −0.36, p < 0.05) (Table 3). A significant inverse correlation was also found between
two-chamber (2B)
strain and EF (r = −0.36, p < 0.05), as well as a positive correlation between 2B strain and left
atrial
volume (r = 0.36, p < 0.05) (Table 5).
In addition, a significant positive correlation was observed between isovolumic relaxation time (IVRT)
and
LAVI
at 3 months after CRT implantation (r = 0.66, p = 0.001) (Table 4). A positive correlation was also
found
between LAVI and E/e′ measured by tissue Doppler (r = 0.53, p < 0.05) (Table 3).
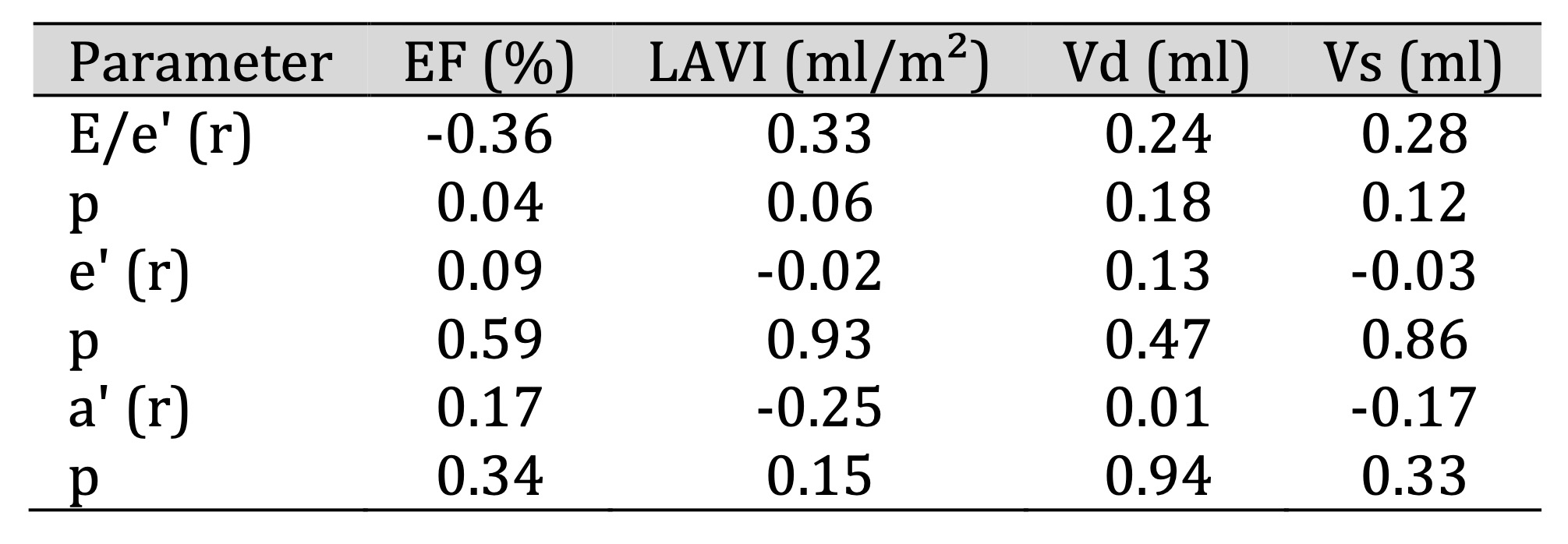
Table 3: Relationship between tissue Doppler echocardiographic parameters and total EF (%), LAVI, Vd, Vs at 3 months after CRT implantation. Comments: At 3 months after implantation: There was a significant inverse correlation between E/e’ ratio and total EF (r = -0.36, p < 0.05)
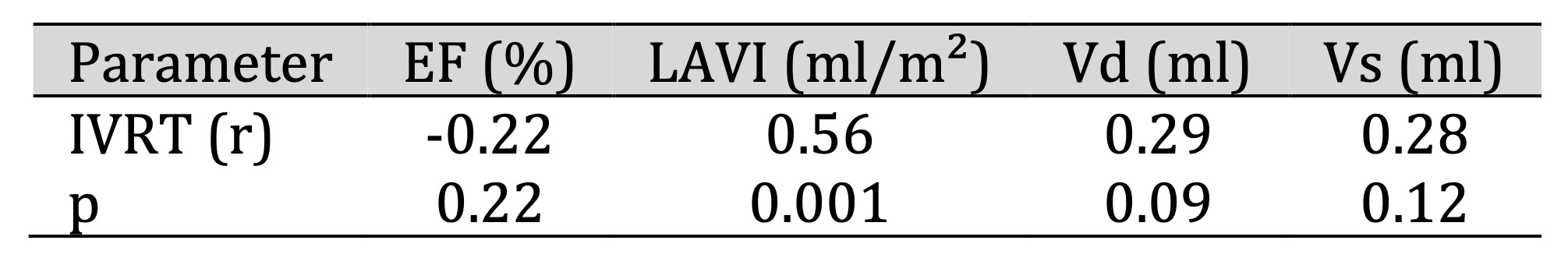
Table 4: Relationship between echocardiographic parameters and isovolumic relaxation time (IVRT) at 3 months after implantation. Comments: At 3 months after implantation: There was a significant positive correlation between IVRT and left atrial volume index (LAVI) (p < 0.05)
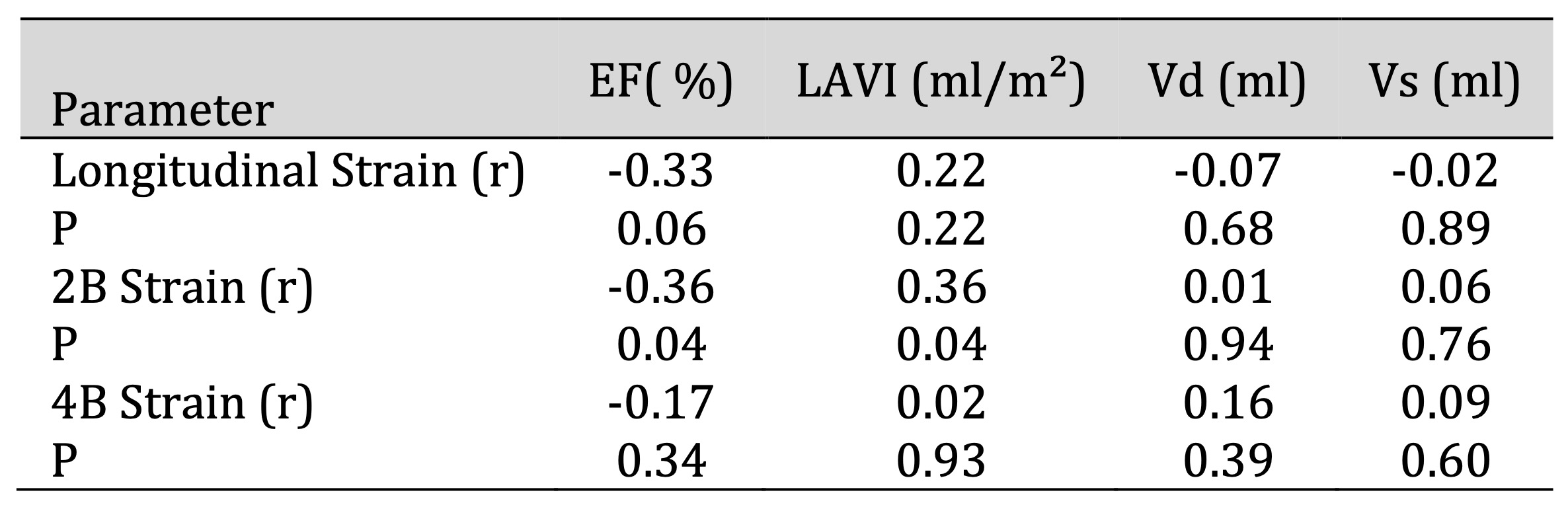
Table 5: Relationship between 2D echocardiographic parameters and myocardial strain at 3 months after implantation. Comments: At 3 months after implantation, there was a significant inverse correlation between 2B strain and total EF (r = -0.36, p < 0.05) and a significant positive correlation with left atrial volume LAV (r=0.36, p < 0.05)
Discussion
Demographic Characteristics
The mean age of patients in this study was 57.8 years, with a higher proportion of patients aged
≥60
years. This
age distribution is consistent with the epidemiology of severe heart failure and reflects the
typical
population
indicated for CRT [1,2].
QRS Duration and Left Ventricular Remodeling
The present study demonstrated a significant association between QRS duration and left
ventricular
remodeling.
QRS narrowing after CRT implantation was associated with reductions in left ventricular volumes
and
improvement
in EF, supporting the concept that electrical resynchronization contributes to reverse
remodeling. These
findings are consistent with previous studies reporting improved ventricular function in
patients with QRS
narrowing after CRT implantation [6–10].
Role of Strain Echocardiography in CRT Assessment
Strain echocardiography provides a sensitive assessment of myocardial deformation and allows
detection of
subtle
functional changes that may not be apparent with conventional echocardiography. The observed
associations
between myocardial strain parameters and QRS recovery highlight the value of strain imaging in
evaluating
CRT
response, particularly in patients with wider QRS complexes [13].
Left Atrial Volume and CRT Response
Left atrial volume index reflects chronic diastolic burden and elevated filling pressures. The
observed
relationship between changes in LAVI and myocardial strain suggests that atrial remodeling
parallels
ventricular
functional improvement following CRT implantation. This finding is in line with previous studies
indicating that
LAVI may serve as a predictor of CRT response [14,19].
Diastolic Function Parameters
The observed relationships between E/e′ ratio, EF, IVRT, and LAVI indicate that diastolic
function
improves
alongside systolic function after CRT implantation. Increased E/e′ ratio and prolonged IVRT were
associated with
impaired ventricular performance, supporting the role of tissue Doppler echocardiography in
monitoring
diastolic
function in heart failure patients undergoing CRT [15–20].
Clinical Implications
Overall, these findings emphasize the complementary role of strain echocardiography and
conventional
echocardiography in assessing left ventricular remodeling and treatment response after CRT.
Echocardiography
remains an indispensable tool for diagnosis, risk stratification, and follow-up in patients with
severe
heart
failure.
Conclusion
This study revealed several relationships between left ventricular remodeling assessed by strain echocardiography and conventional echocardiography before and after CRT in severe heart failure patiens: Left ventricular volume before and after CRT implantation showed significant changes from 1 to 3 months (p < 0.001) and had a strong positive correlation with the QRS complex on the electrocardiogram of patients in the study group (r = 0.65). There was a significant positive correlation between the change in left atrial volume (LAVI) and myocardial 4B strain in the QRS (ms) ≥ 140 group (r = 0.96; p < 0.05). At 3 months after CRT implantation, there was a significant positive correlation between isovolumic relaxation time (IVRT) and left atrial volume (LAVI) (p < 0.05), and between left atrial volume and E/e' parameter measured by tissue Doppler (r = 0.53; p < 0.05). However, there was an inverse correlation between E/e' ratio and total EF (r = -0.36; p < 0.05), between 2B strain and total EF (r = -0.36; p < 0.05), and between 2B strain and left atrial volume LAV (r = 0.36; p < 0.05).
Thus, echocardiography, especially tissue Doppler echocardiography, is an indispensable tool in diagnosing, assessing risk factors, and monitoring heart failure. At 3 months after CRT implantation, there was a significant improvement in left atrial volume and ejection fraction (EF), with statistically significant results indicating better treatment effectiveness after 3 months of CRT implantation.
Limitations And Recommendations
This study still has some limitations that need to be addressed in subsequent, larger-scale
studies on
this
issue: The smal sample size and sample size was not large enough (we recruited 33 patients), but
each
patient
directly underwent echocardiography at 4 time points (before implantation, immediately after
implantation,
1
month after implantation, and 3 months after implantation); The study was conducted at a single
center
(Single-center study); A comparative study with a control group was not performed (Lack of a
control
group); And
the follow-up time was not long enough (Short follow-up period). Therefore, we recommend that
new studies
are
needed on the role of echocardiography in evaluating the effectiveness of heart failure
treatment after
CRT
implantation, with a larger sample size, and potentially conducted as a multi-center study on
this issue.
Abbreviations
CRT ((Cardiac Resynchronization Therapy) - Cardiac resynchronization therapy; Dd (End diastolic diameter) - End-diastolic diameter; Ds (End systolic diameter) - End-systolic diameter; Ao (Aorta) – Aorta; EF (Ejection fraction) - Ejection fraction; NYHA (New York Heart Association) - New York Heart Association classification; Vd (End diastolic volume) - End-diastolic volume; Vs (End systolic volume) - End-systolic volume; VLT (Left Ventricle) - Left Ventricle; LA (Left Atrium) - Left Atrium; LAVi (Left atrium Interventricular) - Left atrial volume index; E/A (Early diastolic filling/Atrial contraction) - Ratio of early diastolic flow velocity and atrial contraction velocity in mitral inflow (E wave and A wave); E/e' - Ratio of early diastolic mitral inflow velocity to early diastolic myocardial velocity at the mitral annulus; e' (tissue Doppler velocity) - Early diastolic myocardial velocity; IVRT (Isovolumic Relaxation Time) - Isovolumic relaxation time; s' (s-prime) - Peak systolic myocardial velocity; a' (a-prime) - Peak late diastolic myocardial velocity; VTI (Velocity Time Integral) - Velocity Time Integral; TAPSE (Tricuspid Annular Plane Systolic Excursion) - Tricuspid Annular Plane Systolic Excursion (TAPSE); IVS - Interventricular septum; GLS (Global Longitudinal Strain) – Global Longitudinal Strain.);
Disclosure Statement
The authors have nothing to disclose.
References
| 1 | McMurray JJ, Adamopoulos S, Anker SD, etal., ESC committee for practice Guidelines. ESC
guideline
for the diagnosis and treatment of acute and chronic heart failure 2012: The Task force
for the
Diagnosis and treatment of Acute and Chronic Heart failure 2012 of the Eropean Society of
Cardiology. Developedi n collaboration with the Heart Failure Association (HFA) ofthe ESC.
Eur Heart
J. 2012; 33:1787-1847.
|
| 2 | Chu SD, Doan QH, Tran MT, et al. Individualized treatment strategy for acute
exacerbation of
chronic heart failure in patient with dilated cardiomyopathy: What is the optimal
treatment? Pak
Heart J 2024; 57 (01): 192-198.
|
| 3 | Dought RN, Whalley GA, Walsh HA et al. Effect of carvedilolon out come aftermyocardial
in farction
in patients with left ventricular dysfunction: the CAPRICORN randomisedtrial. Lancet 2001;
357:
1385-1390.
https://doi.org/10.1016/S0140-6736(00)04560-8 |
| 4 | Epstien EA, DiMarco JP, etal., ACC/AHA/HRS 2008 guidelines for Device-Based therapy of
cardiac
Rhythm Abnormallities; JACC. 2008; 21: 1-62.
https://doi.org/10.1016/j.jacc.2008.02.032 |
| 5 | Saunders BD,Trapp RG., Basic& CliniccalBiostatisstics 2n. Prentice - HallInternational
Inc. 1994.
|
| 6 | Rickard J, Popovic Z, Verhaert D, Sraow D, Baranowski B, Martin DO, Lindsay BD, Varma N,
Tchou P,
Grimm RA, Wilkoff BL, Chung MK. The QRS narrowing index predicts reverse left ventricular
remodeling
following cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2011
May;34(5):604-11. doi:
10.1111/j.1540-8159.2010.03022.x. Epub 2011 Jan 28
https://doi.org/10.1111/j.1540-8159.2010.03022.x |
| 7 | De Winter O, Van de Veire N, Van Heuverswijn F, Van Pottelberge G, Gillebert TC, De
Sutter J.
Relationship between QRS duration, left ventricular volumes and prevalence of nonviability
in
patients with coronary artery disease and severe left ventricular dysfunction. Eur J Heart
Fail.
2006 May;8(3):275-7 doi: 10.1016/j.ejheart.2005.10.001. Epub 2005 Nov 21
https://doi.org/10.1016/j.ejheart.2005.10.001 |
| 8 | Guo Z, Liu X, Cheng X, Liu C, Li P, He Y, Rao R, Li C, Chen Y, Zhang Y, Luo X, Wang J.
Combination
of Left Ventricular End-Diastolic Diameter and QRS Duration Strongly Predicts Good
Response to and
Prognosis of Cardiac Resynchronization Therapy. Cardiol Res Pract. 2020 Jan
17;2020:1257578. doi:
10.1155/2020/1257578.
https://doi.org/10.1155/2020/1257578 |
| 9 | Rickard J, Baranowski B, Grimm RA, Niebauer M, Varma N, Tang WHW, Wilkoff BL. Left
Ventricular
Size does not Modify the Effect of QRS Duration in Predicting Response to Cardiac
Resynchronization
Therapy. Pacing Clin Electrophysiol. 2017 May;40(5):482-487. doi: 10.1111/pace.13043.
https://doi.org/10.1111/pace.13043 |
| 10 | Rickard J, Popovic Z, Verhaert D, Sraow D, Baranowski B, Martin DO, Lindsay BD, Varma N,
Tchou P,
Grimm RA, Wilkoff BL, Chung MK. The QRS narrowing index predicts reverse left ventricular
remodeling
following cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2011
May;34(5):604-11. doi:
10.1111/j.1540-8159.2010.03022.x. Epub 2011 Jan 28.
https://doi.org/10.1111/j.1540-8159.2010.03022.x |
| 11 |
https://www.itmedicalteam.pl/articles/impact-of-cardiac-resynchronizationtherapy-on-heart-failure-patientsexperience-from-one-center-103959.html
|
| 12 | De Winter O, Van de Veire N, Van Heuverswijn F, Van Pottelberge G, Gillebert TC, De
Sutter J.
Relationship between QRS duration, left ventricular volumes and prevalence of nonviability
in
patients with coronary artery disease and severe left ventricular dysfunction. Eur J Heart
Fail.
2006 May;8(3):275-7.doi: 10.1016/j.ejheart.2005.10.001.
https://doi.org/10.1016/j.ejheart.2005.10.001 |
| 13 | Bax JJ, Delgado V, Sogaard P, Singh JP, Abraham WT, Borer JS, Dickstein K, Gras D,
Brugada J,
Robertson M, Ford I, Krum H, Holzmeister J, Ruschitzka F, Gorcsan J. Prognostic
implications of left
ventricular global longitudinal strain in heart failure patients with narrow QRS complex
treated
with cardiac resynchronization therapy: a subanalysis of the randomized EchoCRT trial. Eur
Heart J.
2017 Mar 7;38(10):720-726. doi: 10.1093/eurheartj/ehw506.
https://doi.org/10.1093/eurheartj/ehw506 |
| 14 | https://journal.chestnet.org/article/S0012-3692(16)44197-8/fulltext
|
| 15 | Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial
stiffening in
patients with heart failure and preserved ejection fraction: implications for systolic and
diastolic
reserve limitations. Circulation. 2003 Jul 8;107(26):351-7.doi:
10.1161/01.CIR.0000065421.30461.8B.
https://doi.org/10.1161/01.CIR.0000048123.22359.A0 |
| 16 | Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, González A, Herregods MC,
Fagard RH,
Diez J, Staessen JA. Prevalence of left ventricular diastolic dysfunction in a general
population.
Circ Heart Fail. 2009 Jan;2(1):105-12 doi: 10.1161/CIRCHEARTFAILURE.108.822011 Epub 2008
Nov 25.
https://doi.org/10.1161/CIRCHEARTFAILURE.108.822627 |
| 17 | Sengupta PP, Krishnamoorthy VK, Korinek J, Narula J, Vannan MA, Lester SJ, Tajik JA,
Seward JB,
Khandheria BK, Belohlavek M. Left ventricular form and function revisited: applied
translational
science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr. 2007
Nov;20(11):539-51. doi:
10.1016/j.echo.2007.02.014.
https://doi.org/10.1016/j.echo.2007.02.014 |
| 18 | Reant P, Labrousse L, Lafitte S, Bordachar P, Pillois X, Tariosse L, Bonoron-Adele S,
Padois P,
Deville C, Roudaut R, Dos Santos P. Experimental validation of circumferential,
longitudinal, and
radial 2-dimensional strain during dobutamine stress echocardiography in ischemic
conditions. J Am
Coll Cardiol. 2008 Jan 1;51(1):149-57. doi: 10.1016/j.jacc.2007.08.055.
https://doi.org/10.1016/j.jacc.2007.08.055 |
| 19 | Møller JE, Hillis GS, Oh JK, Reeder GS, Gersh BJ, Pellikka PA. Left atrial volume: a
powerful
predictor of survival after acute myocardial infarction. Circulation. 2003 Aug
5;107(30):2207-12.
doi: 10.1161/01.CIR.0000066314.99751.68.
https://doi.org/10.1161/01.CIR.0000066318.21784.43 |
| 20 | Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon
MA,
Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by
cardiovascular magnetic resonance is increased in diabetes and associated with mortality
and
incident heart failure admission. Eur Heart J. 2014 Dec 21;35(10):657-64.doi:
10.1093/eurheartj/eht174.
https://doi.org/10.1093/eurheartj/eht174 |