Original Article - DOI:10.33594/000000659
Accepted 12 August 2023 - Published online 1 September 2023
High Fat High Sugar Diet Reduces Small Intestinal Secretion by Sex-Dependent Mechanisms
bCollege of Veterinary Medicine,
cDepartment of Physiology: College of Graduate Studies, Midwestern University, Glendale, Arizona, USA.
Keywords
Abstract
Background/Aims:
The goal of this study was to determine the influence of high-fat high-sugar diet (Western diet) on intestinal function and subsequently to determine if there were any beneficial effects of exercise, genistein (a naturally occurring phytoestrogen) or both, on the intestine.Methods:
We measured transepithelial short circuit current (Isc), across freshly isolated segments of jejunum from male and female C57Bl/6J mice randomly assigned to one of the following groups for the 12-week study duration: high-fat high-sugar diet (HFS), HFS with genistein (Gen), HFS with exercise (Ex), or HFS with both genistein and exercise (Gen+Ex) and compared them to lean controls. Genistein concentration was 600 mg genistein/kg diet. Exercise comprised of moderate intensity treadmill running (150 min per week). At the completion of the study, segments of jejunum were frozen for western blot determination of key proteins involved in secretory and absorptive functions, as well as senescence. Intestinal morphology was assessed. Serum cytokine assays were performed.Results:
Basal Isc was significantly decreased (by 70%, P <0.05) in HFS females and males versus leans. This decrease was partially mitigated by exercise in both sexes. In females, the HFS-induced decrease in Isc was attributed to a significant loss of CLC2, NKCC1 and CFTR expression whereas in males this was due to a significant loss of Na/K-ATPase, KCa and NKCC1 expression (indicating sex-dependent mechanisms). Exercise mitigated most of the loss of Isc in both sexes. Our data suggested that A2BR levels were dysregulated in HFS fed mice and that concomitant treatment with Gen or Gen+Ex prevented this disruption in females only. Inflammatory state was associated with body weight changes.Conclusion:
Our data suggests that the reduced basal jejunal Isc in HFS mice is attributed to sex-dependent mechanisms and while exercise partially mitigated this, it’s mechanism of action was unclear. Improved understanding of Western diet induced intestinal dysfunctions may allow for the development of novel drug targets to treat gastrointestinal disturbances in diabetic obesity.Introduction
Metabolic syndrome includes a plethora of symptoms including obesity and type 2 diabetes and thus presents an increasing global healthcare burden [1]. Obesity has been linked to chronic gastrointestinal dysfunction (dyspepsia, constipation, or diarrhea) and specifically females with high BMI have been associated with increased prevalence of diarrhea [2]. In obese children, a higher incidence of constipation has been assessed without associated delays in colonic motility [3].
High-fat diet models have been utilized to further understand the etiology and consequences of metabolic syndrome on gastrointestinal function. For example, high fat diet consumption has been proposed to increase fat content within enterocytes, decreasing the expression of nutrient transporters GLUT2 and PEPT1, likely a consequence of reducing PKA and PKC-mediated intracellular pathways [4]. Other studies have shown that a high-fat diet induces changes in the enteric nervous system of the small intestine [5, 6] and disrupts the intestinal epithelial barrier [7].
We have previously shown that genistein (a naturally occurring phytoestrogen found in soy, [8]) fed to C57BL/6J lean mice (600 mg genistein/kg diet) for a 4-week duration, generates serum genistein levels of approximately 4-7 µM in male and female mice [9]. Interestingly, consumption of soy milk in humans generates comparable serum levels of genistein, indicating that our dose is readily achievable within the population [10]. We have also shown that dietary exposure of the same genistein concentration (600 mg genistein/kg diet) for 4-weeks, prevents intestinal dysfunction in the ob/ob murine model of diabetic obesity, improving basal intestinal Isc, mediated by sex-dependent changes in expression of key ion transporters [11].
The influence of exercise on gastrointestinal function is a relatively understudied area. High intensity exercise has been proposed to result in gastrointestinal damage, assessed via changes in intestinal fatty acid binding protein [12]. In contrast, moderate exercise training has been shown to provide several more positive effects; (1) improved gastrointestinal function (reducing bouts of diarrhea) in intensive care patients [13], (2) reduced numbers of apoptotic cells in pancreas removed from exercise trained-high fat diet fed mice [14], and (3) protection of intestinal barrier function from high-fat diet induced permeabilization [15, 16].
The goal of the current study was to ascertain whether dietary genistein, moderate exercise, or both combined, would alter jejunum physiology in a high-fat high-sugar diet (HFS) model of diabetic obesity. To that end, we evaluated jejunum transepithelial short circuit current (Isc, a measure of Cl- secretion) and key transporters associated with maintaining appropriate Cl- secretory function. In addition, we measured expression of key proteins involved in the jejunum’s absorptive role (namely, SGLT-1, GLUT2, GLUT5). Moreover, since a western diet induces chronic inflammation, we assessed levels of serum inflammatory cytokines, intestinal senescence, and intestinal morphology. Improved understanding of the intestinal dysfunctions resulting from chronic consumption of western diets (HFS) may allow for the development of novel drug targets to alleviate intestinal problems associated with metabolic syndrome.
Materials and Methods
Mouse model of diet induced diabetic obesity
Male and female C57BL/6 mice (4 weeks old) were purchased from Charles River Laboratory (Wilmington, MA). Mice were acclimated for a period of 1 week and then randomly divided into five diet groups for a period of 12 weeks: standard rodent diet Harlan 5001 (std) with normal water, high-fat diet (HFS, 60% fat), high fat diet + genistein (HFS+Gen, 600 mg genistein/kg high fat diet), high fat diet + exercise (HFS+Ex), high fat diet + genistein + exercise (HFS+Gen+Ex). All mice fed high fat diet were also exposed to sugar in the drinking water (42 g/L; 55% fructose/45% sucrose, F/S) throughout the 12-week diet study. All mice were given food and water ad libitum. The high-fat diet, with or without genistein, was purchased from Dyets Inc (Bethlehem, PA). Exercised mice were exposed to running on a treadmill for 5 days/week, a total of 150 min/week from week 3-12, as previously described [17]. The moderate intensity exercise protocol was as follows: week 1 (10 min at 10 m/min), week 2 (20 min at 10 m/min), week 3 (30 min at 12 m/min), weeks 4-12 (30 min at 15 m/min). Mice were housed two per cage in an animal care facility with 12:12-hour light-dark cycle. Each week, body weight and overall health was assessed. At completion of the diet study, mice were euthanized by asphyxiation in an atmosphere of 100% CO2, followed immediately by surgical thoracotomy (inducing pneumothorax). Animal care was conducted in accordance with established guidelines, and all protocols were approved by the Midwestern University Institutional Animal Care and Use Committee.
Bioelectric measurement of intestinal secretion
Assessments of transepithelial short circuit current (Isc, μA/cm2) were obtained as described previously [11, 18], with 0.3 cm2 exposed surface area of freshly excised jejunum. Short circuit current was measured via an automatic voltage clamp (VCC-600, Physiologic Instruments, San Diego, CA) [11, 18]. All tissue segments were maintained in 1 μM indomethacin which served to reduce the tissue exposure to endogenously generated prostanoids due to tissue handling [19]. Variations in intrinsic intestinal neural tone were reduced by exposing the serosal side of the tissues to tetrodotoxin (0.1 μM) which also served to limit the absorptive capacity of the mucosa [20]. To prevent an inward current due to Na+-coupled glucose transport glucose (10 mM) was added to the serosal KBR bath and mannitol (10 mM) substituted for glucose in the mucosal KBR bath [21]. Experimental protocols. Tissues were exposed to KBR (20 min) and steady-state basal Isc measured at that time. At time 20 min, we added forskolin (10 μM, bilateral) until steady state was achieved at time 40 minutes (i.e., a further 20 min later), then bumetanide (100 μM, serosal) for 10 minutes. Glucose (10 mM, mucosal) was added at the end of each experiment to stimulate Na+-coupled glucose transport, as an assessment of tissue viability (denoted by > 10% increase in Isc). Any tissue that demonstrated a failure to respond to glucose within this parameter was discarded from the study. Solutions. Cl- containing KBR contained the following (in mM): 115 NaCl, 25 NaHCO3, 5 KCl, 1.2 MgCl2 and 1.2 CaCl2, pH 7.4.
Histology and morphology with H & E staining
Freshly isolated jejunum was embedded and flash frozen in Optimal Cutting Temperature compound (OCT, Tissue-Tek, Torrance, CA). Frozen sliced sections (10 µm) of jejunum were stained with a standard protocol for hematoxylin and eosin (H & E) staining, as previously published, [11, 18, 22]. Measures of villi length, crypt depth, and wall thickness were taken using Image J (NIH), from images taken at 10x magnification. Averages of measurements were taken from 5-8 separate slices per frozen section of jejunum (per mouse) and data are presented as the average of multiple mice per group.
Western blot analysis
At euthanasia, segments of jejunum were immediately snap frozen in liquid nitrogen and stored at -80°C. Jejunum was later prepared for western blot analysis as described previously [11, 22, 23]. Blots were individually incubated with each of the following primary antibodies overnight at 4 ºC: Na/K-ATPase (1:1000, Cell Signaling, Danvers, MA), KCa and CLC2 (1:500, Alomone labs, Jerusalem), A2BR (1:1000, Abcam, Cambridge, MA), CREB (1:1000, Cell Signaling, Danvers, MA), GLUT2 (1:500, Santa Cruz, Dallas, TX), GLUT5 (1:500, Millipore, Burlington, MA), SGLT1 (1:500, Abcam, Cambridge, MA), p-H2AX and P53 (1:1000, Cell Signaling, Danvers, MA). Blots were either re-probed for actin (anti-actin primary antibody, 1:4000, Thermo Scientific, Rockford, IL) or re-probed for GAPDH (anti-GAPDH primary antibody, 1:4000, Thermo Scientific, Rockford, IL) for 1 hour at room temperature, to serve as the control. Blots were washed and then incubated with the appropriate secondary antibody anti-mouse IgG (H+L) (1:15, 000, Dylight, Thermo Scientific Rockford, IL). After washing, blots were incubated with secondary antibody, anti-rabbit IgG (H+L) Dylight (1:15, 000, Thermo Scientific, Rockford, IL), for 1 hour at room temperature. Images of membranes were taken with all proteins of interest normalized to either actin or GAPDH. Band density was analyzed using Odyssey-Clx (LI-COR, Lincoln, NE) and Image Studio (LI-COR, Lincoln, NE).
PCR analysis
Segments of jejunum were immediately snap frozen in liquid nitrogen and stored at -80°C. Jejunum was later prepared for PCR analysis as follows: tissue was homogenized in Rhino Tubes (Next Advance. Raymertown, NY) in a Bullet Blender using the standard protocol. RNA was extracted from tissue with the Invitrogen PureLink™ RNA Mini Kit (Thermo-Fisher Inc, Wiltham, MA) using the standard protocol. The RNA was reverse transcribed with iScript™ Reverse Transcription Supermix (BioRad Hercules, CA) using the standard protocol. DNA products were diluted 1:1000 then used to perform a qPCR reaction with iQ SYBR® Green Supermix (BioRad) in a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Thermo-Fisher) using the primers described below. All genes were run for 35 cycles with an annealing temperature of 60°C. The target gene was compared to GAPDH and quantified using standard methods. The following primers were used (Integrated DNA technologies, Coralville, Iowa): NKCC1; Forward: ATTGTAAGATCCGAGTATTCATTGG and Reverse: GAGAAGTCTATCCGGAATTTACTCA and CFTR; Forward: TCAAGATACCCCCGGTGACA and Reverse: CGCACCAAATCAGCACTAGC.
Serum Measures
Serum samples were assayed for the following: glucose (Wako Diagnostics, Mountain View, CA), insulin (Alpco Diagnostics, Salem, NH), cytokines (Milliplex Mouse Cytokine Bead Panel (EMD Millipore Corporation, Billerica, MA) and Fatty acid binding protein 1, FABP1 (R&D Systems Inc. Minneapolis, MN),
Statistics. Data are expressed as mean ± SEM. Numbers in parentheses represent numbers of tissues used from separate individual mice. One-way ANOVA with Dunnett’s multiple comparison test was performed using GraphPad (San Diego, CA) and P <0.05 was considered statistically significant.
Results
Ussing chamber bioelectric measurements
To best determine the effect of high-fat and high-sugar diet on jejunum epithelial short circuit current (transepithelial Cl- secretion, Isc) we examined freshly excised jejunum in the absence and presence of agonist stimulation.
As shown in Fig. 1A, C, basal Isc was significantly decreased in HFS-fed female mice (19.6 ± 5.4 μA/cm2, n = 5, P<0.05) when compared to lean controls (67.06 ± 6.18 μA/cm2, n = 5). Ex alone partially mitigated this in females (43.3 ± 6.0 μA/cm2, n = 5, P<0.05). Neither Gen alone (23.7 ± 2.4 μA/cm2, n = 5) nor Gen+Ex (21.6 ± 6.5 μA/cm2, n = 5) showed any effect on basal Isc. Basal Isc was similarly significantly decreased in HFS-fed male mice (19.3 ± 1.7 μA/cm2, n = 5, P<0.05) when compared to lean controls (66.8 ± 8.1 μA/cm2, n = 5). Ex alone (42.4 ± 6.6 μA/cm2, n = 5, P<0.05, Fig. 1B, C), and Gen alone partially mitigated this decrease in males (31.4 ± 4.6 μA/cm2, n = 5, P<0.05, Fig. 1B, C ).
The cAMP stimulated Isc was measured following application of forskolin (bilateral, 10 μM, Fig. 1A, 1B, D ). The forskolin-mediated increase in Isc was significantly reduced by HFS in both females and males when compared to lean controls. While there was no treatment effect in females, however Gen alone induced a significant 1.8-fold increase in forskolin-stimulated Isc in males (Fig. 1D ). The bumetanide-inhibited Isc was significantly reduced by HFS-diet in both females and males (Fig. 1E ) but showed no significant treatment effects. These data suggest that the diabetic-obese, HFS-fed mice have decreased jejunum basal Isc (partially recovered by exercise in females, and either exercise or genistein in males) and moreover, the diabetic-obese mouse has a diminished response to cAMP-mediated Isc (partially recovered by genistein in males).
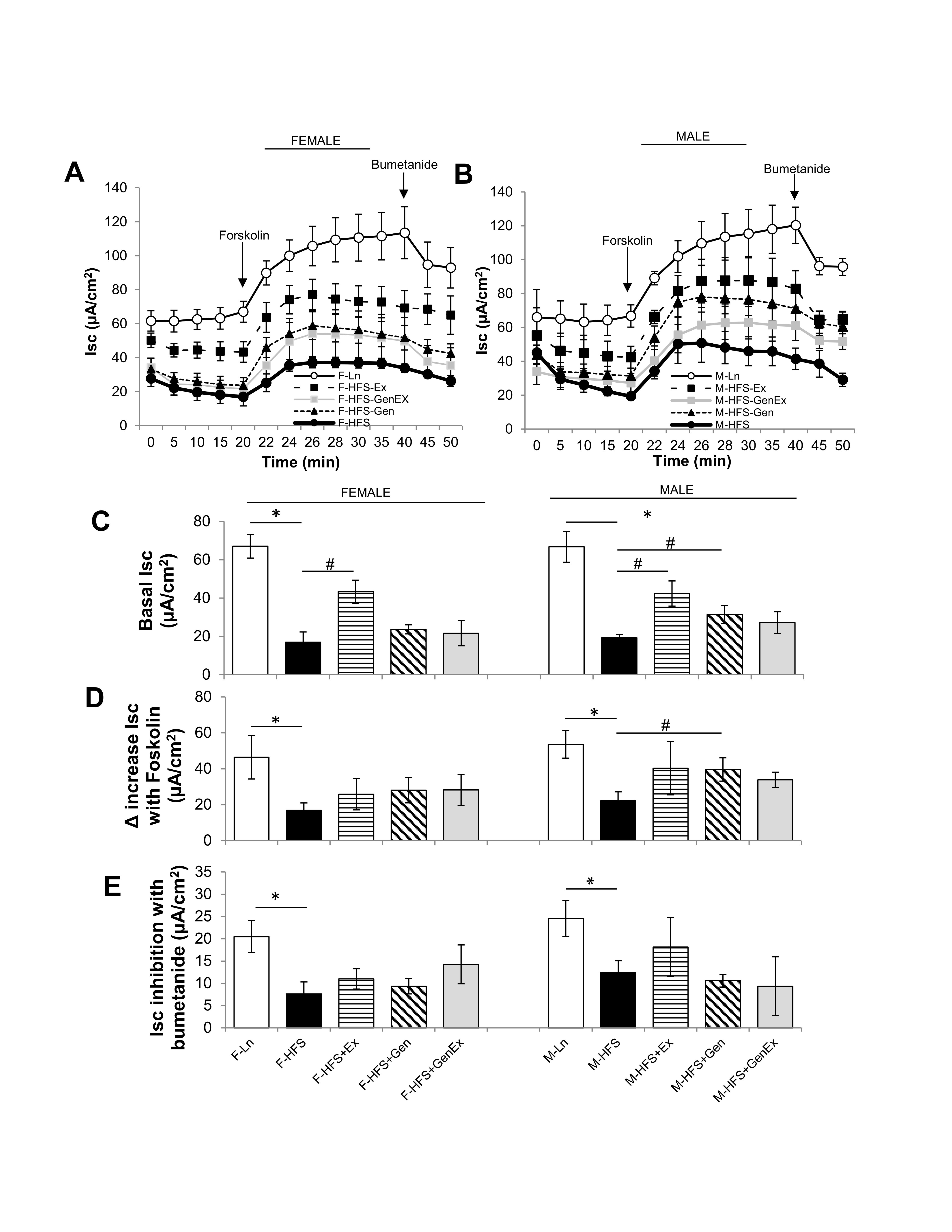
Fig. 1: Effect of high fat/high sugar diet, genistein and exercise on Isc. (A) Average raw trace data of recordings from jejunum from female mice (n = 5/group). (B) Average raw trace data of recordings from jejunum from male mice (n = 5/group). Controls, standard diet (open circle, Ln), high fat high sugar diet (solid circle, HFS), HFS + exercise (solid square, Ex), HFS + genistein (solid triangle, Gen), HFS + genistein + exercise (gray square, GenEx). Basal Isc recorded from time 0-20 mins, forskolin (10 μM, bilateral) added at time 20 mins, bumetanide (100 μM, basolateral) added at 40 mins. (C) Average basal Isc at time 20 mins. (D) Average increase in Isc with forskolin (10 μM, bilateral). (E) Average inhibition in Isc with bumetanide (100 μM, basolateral). Data are means ± SEM (n = 4-8/group). * denotes P < 0.05, statistical difference to lean controls, and # denotes P < 0.05, statistical treatment effect.
Evaluation of key proteins involved in jejunum chloride secretion and nutrient absorption
Small intestinal secretion requires the interplay of several key proteins on the apical and basolateral membranes of the crypt cells; Cl- enters into intestinal epithelial cells via the Na+/K+/2Cl- (NKCC1) co-transporter, activation of apical Cl- channels (such as CFTR) affords a pathway for Cl- exit, basolateral K+ channels maintain the driving force for apical Cl- secretion), and Na+/K+-ATPase activity (responsible for maintaining the Na+ and K+ concentration gradients across the membrane). Total protein expression of Na+/K+-ATPase, KCa, ClC2 were evaluated to assess their contribution towards the reduced basal Isc noted in the HFS fed mice compared to lean counterparts. Expression of total Na+/K+-ATPase protein was comparable in all female groups. Na+/K+-ATPase protein expression was significantly reduced 8-fold in the HFS male mice (0.08 ± 0.07, n = 5, P <0.05) compared to lean controls (0.66 ± 0.18, n = 5), and Ex mitigated this (0.61 ± 0.21, n = 5, P <0.05, Fig. 2A ). Expression of total KCa protein was comparable in all female groups. However, in males, KCa protein expression was significantly reduced 3.4-fold in the HFS fed mice (0.18 ± 0.09, n = 7, P <0.05) compared to lean controls (0.62 ± 0.15, n = 7), and Gen+Ex mitigated this (0.61 ± 0.21, n = 7, P <0.05, Fig. 2B ). Expression of total ClC2 protein was significantly reduced 4.7-fold in the HFS mice (0.11 ± 0.04, n = 5, P <0.05) compared to lean controls (0.54 ± 0.16, n = 5), and Gen mitigated this (0.65 ± 0.16, n = 5, P <0.05, Fig. 2C ). However, in males, ClC2 protein expression was comparable. Assessment of CFTR mRNA levels in female mice indicated an HFS-induced significant decrease (0.49 ± 0.04, n = 4, P <0.05, Fig. 2D ) compared to lean controls (0.89 ± 0.11, n = 4). Treatment groups, however, were without effect compared to the HFS group (no changes noted among the groups in male mice). Assessment of NKCC1 mRNA levels in female mice suggested an HFS-induced significant decrease (0.04 ± 0.01, n = 4, P <0.05, Fig. 2E ) compared to lean controls (0.51 ± 0.19, n = 4) and treatments were without effect. NKCC1 mRNA levels in male mice were similarly significantly decreased by HFS diet (0.24 ± 0.05, n = 5, P <0.05, Fig. 2E ) compared to lean controls (0.66 ± 0.18, n = 5) and treatments were without effect. A schematic of the key transporters involved in generating Isc (and described above) is shown in Fig. 2F .
Expression of total A2BR protein was significantly increased 5.4-fold in female mice fed HFS (0.77 ± 0.11, n = 5, P <0.05) compared to lean controls (0.13 ± 0.06, n = 6). Gen (0.31 ± 0.17, n = 6, P <0.05) and Gen+Ex mitigated this diabetes-induced increased expression (0.22 ± 0.06, n = 5, P <0.05, Fig. 3A ). Total A2BR protein expression was also significantly increased in HFS fed male mice (0.51 ± 0.14, n = 6, P <0.05) compared to lean controls (0.02 ± 0.01, n = 5, Fig. 3A ), and treatments were without effect. Expression of total CREB protein was significantly increased 2.5-fold in female mice fed HFS (0.74 ± 0.15, n = 7, P <0.05) compared to lean controls (0.30 ± 0.12, n = 7), and also significantly increased 8-fold in male mice fed HFS (0.30 ± 0.09, n = 5, P <0.05) compared to lean controls (0.05 ± 0.01, n = 5, Fig. 3B ) and treatments were without effect for both sexes.
Small intestinal absorption of monosaccharide products of digestion (glucose, galactose, and fructose) is mediated across jejunal epithelium into the enterocytes via specific transporters. This absorption is dependent upon key transporters such as the Na+/glucose co-transporter (SGLT1 transporting glucose and galactose on the apical membrane), the facilitated diffusion glucose transporters (GLUT2 on the basolateral membrane and GLUT5 transporting fructose on the apical membrane) and PEPT1 (i.e., proton coupled solute carrier 15, SLC15A1, responsible for the absorption of small peptides across the apical membrane). Therefore, to assess alterations in the jejunum absorptive processes in HFS-fed mice compared to lean counterparts, total GLUT2, GLUT5, SGLT-1 protein expression was quantified using standard western blot techniques. As shown in Fig. 4A, total GLUT2 protein expression, was comparable in all groups of female and male mice (however, there was a trend for an exercise-mediated decrease in males). The amount of total GLUT5 protein expressed in jejunum was comparable in HFS-fed groups compared to lean controls (for both sexes). However, in there was an Ex-induced 2-fold decrease in expression in female mice (0.39 ± 0.07, n = 7, P < 0.05) compared to HFS-fed controls (0.81 ± 0.09, n = 6, Fig. 4B ). Total SGLT-1 protein expression was comparable in all female groups. However, in males total SGLT-1 protein expression increased 1.8-fold with HFS (0.79 ± 0.09, n = 7, P < 0.05) compared to lean controls (0.43 ± 0.15, n = 6, Fig. 4C ), which was prevented by exercise (0.50 ± 0.06, n = 8, Fig. 4C, P < 0.05).
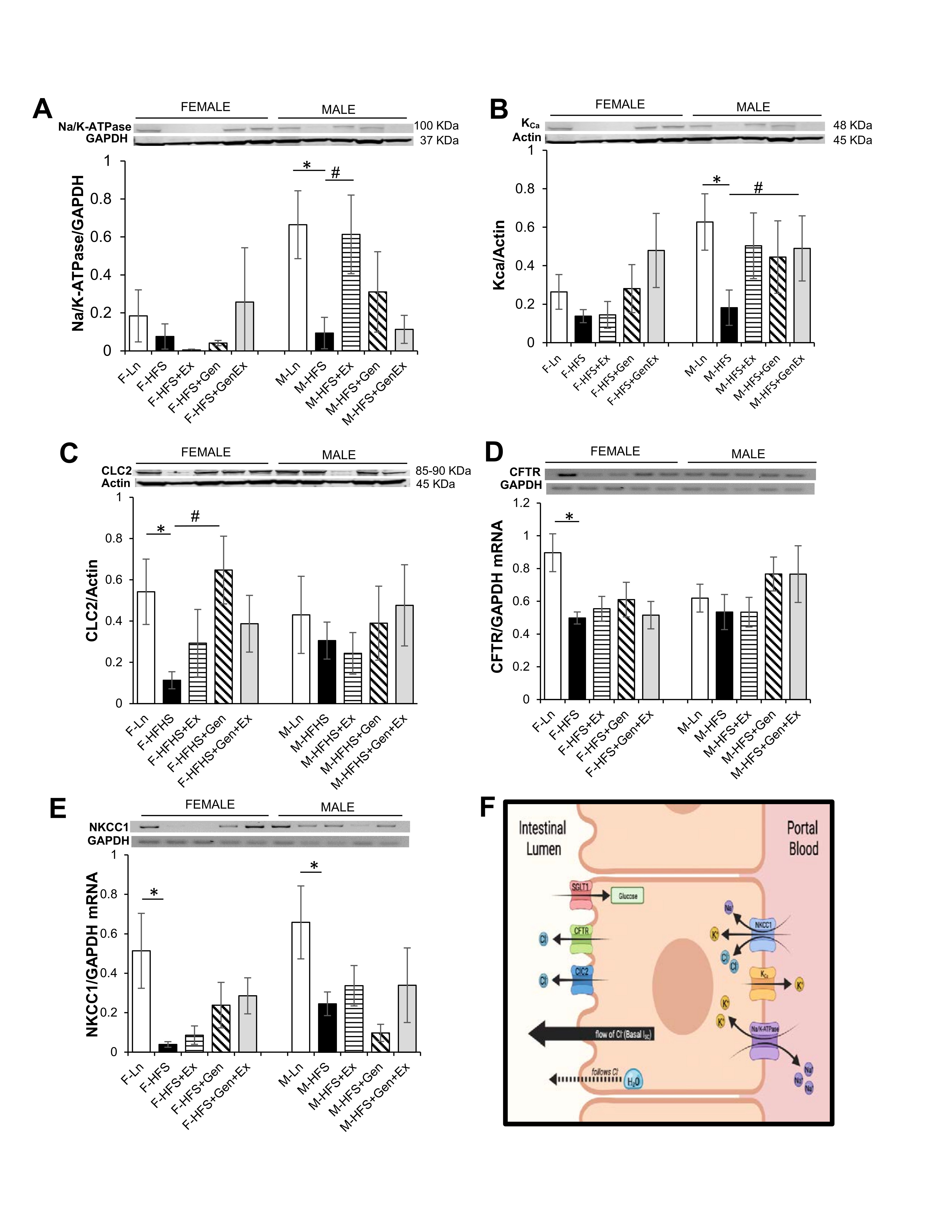
Fig. 2: Effect of high fat/high sugar diet, genistein and exercise on total expression of proteins involved in secretion in jejunum. A. Typical western blot demonstrating Na/K-ATPase expression (normalized to Actin). Average Na/K-ATPase /Actin ratio is shown for all diet groups (n = 4-5/group). B. Typical western blot demonstrating KCa expression (normalized to Actin). Average KCa/Actin ratio is shown for all diet groups (n = 4-7/group). C. Typical western blot demonstrating CLC2 expression (normalized to Actin). Average CLC2/Actin ratio is shown for all diet groups (n = 5-7/group). D. Typical PCR-assessed CFTR mRNA expression (normalized to GAPDH). Average NKCC1/GAPDH ratio is shown for all diet groups (n = 4-5/group). E. Typical PCR assessed NKCC1 mRNA expression (normalized to GAPDH). Average NKCC1/GAPDH ratio is shown for all diet groups (n = 4-5/group). F. Schematic of a typical intestinal crypt cell with the key transporters involved in generating chloride secretion (image created with Biorender.com). Note: Controls, standard diet (Ln, open bars), high fat high sugar diet (HFS, solid black bars), HFS + exercise (HFS+Ex, horizontal line bars), HFS + genistein (HFS+Gen, hashed bars), HFS + genistein + exercise (HFS+Gen+Ex, gray bars). Data are means ± SEM. * denotes P<0.05, statistical difference to lean controls, and # denotes P<0.05, statistical treatment effect.
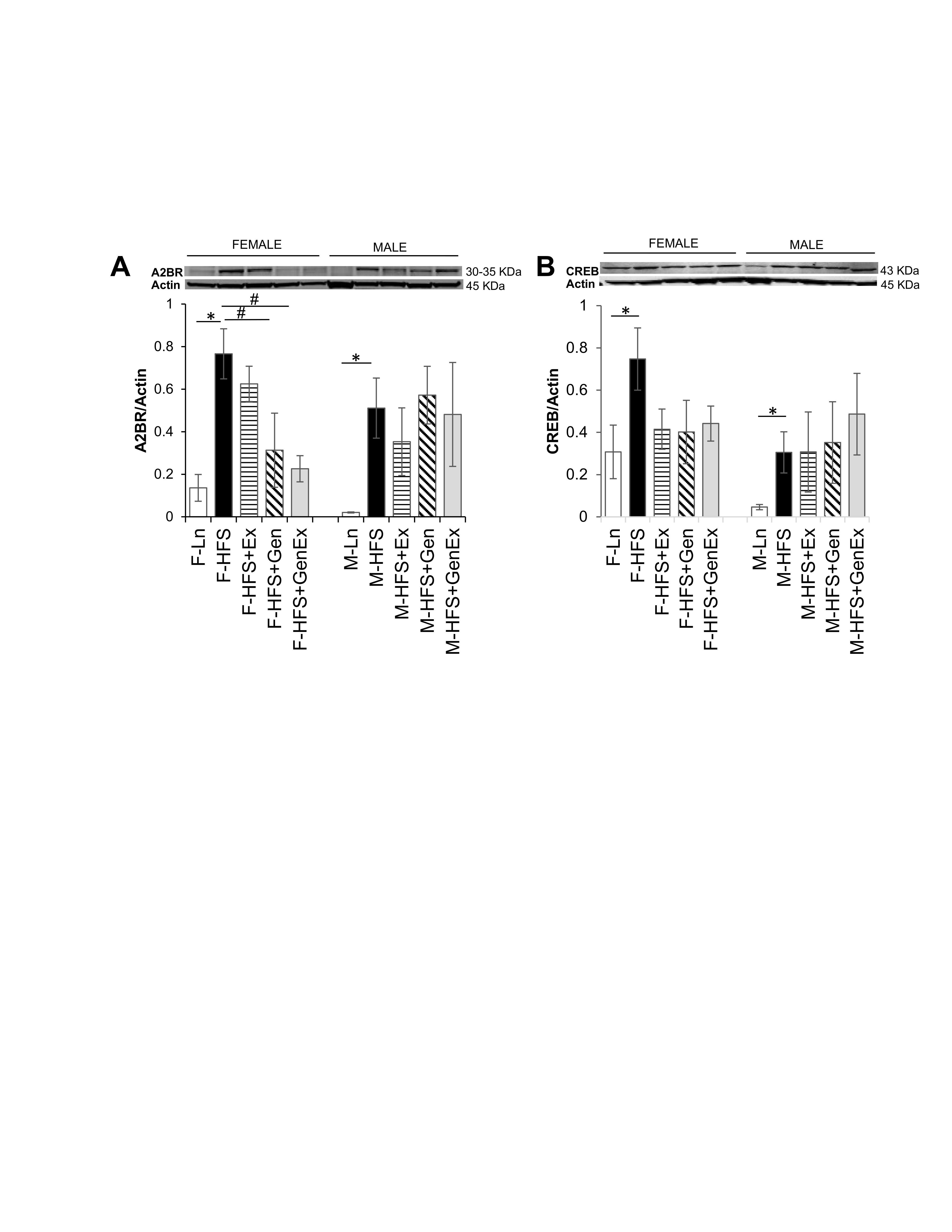
Fig. 3: Effect of high fat/high sugar diet, genistein and exercise on total expression of A2BR and CREB proteins in jejunum. A. Typical western blot demonstrating A2BR expression (normalized to Actin). Average A2BR/Actin ratio is shown for all diet groups (n = 5-6/group). B. Typical western blot demonstrating CREB expression (normalized to Actin). Average CREB/Actin ratio is shown for all diet groups (n = 5-7/group). Note: Controls, standard diet (Ln, open bars), high fat high sugar diet (HFS, solid black bars), HFS + exercise (HFS+Ex, horizontal line bars), HFS + genistein (HFS+Gen, hashed bars), HFS + genistein + exercise (HFS+Gen+Ex, gray bars). Data are means ± SEM. * denotes P < 0.05, statistical difference to lean controls.
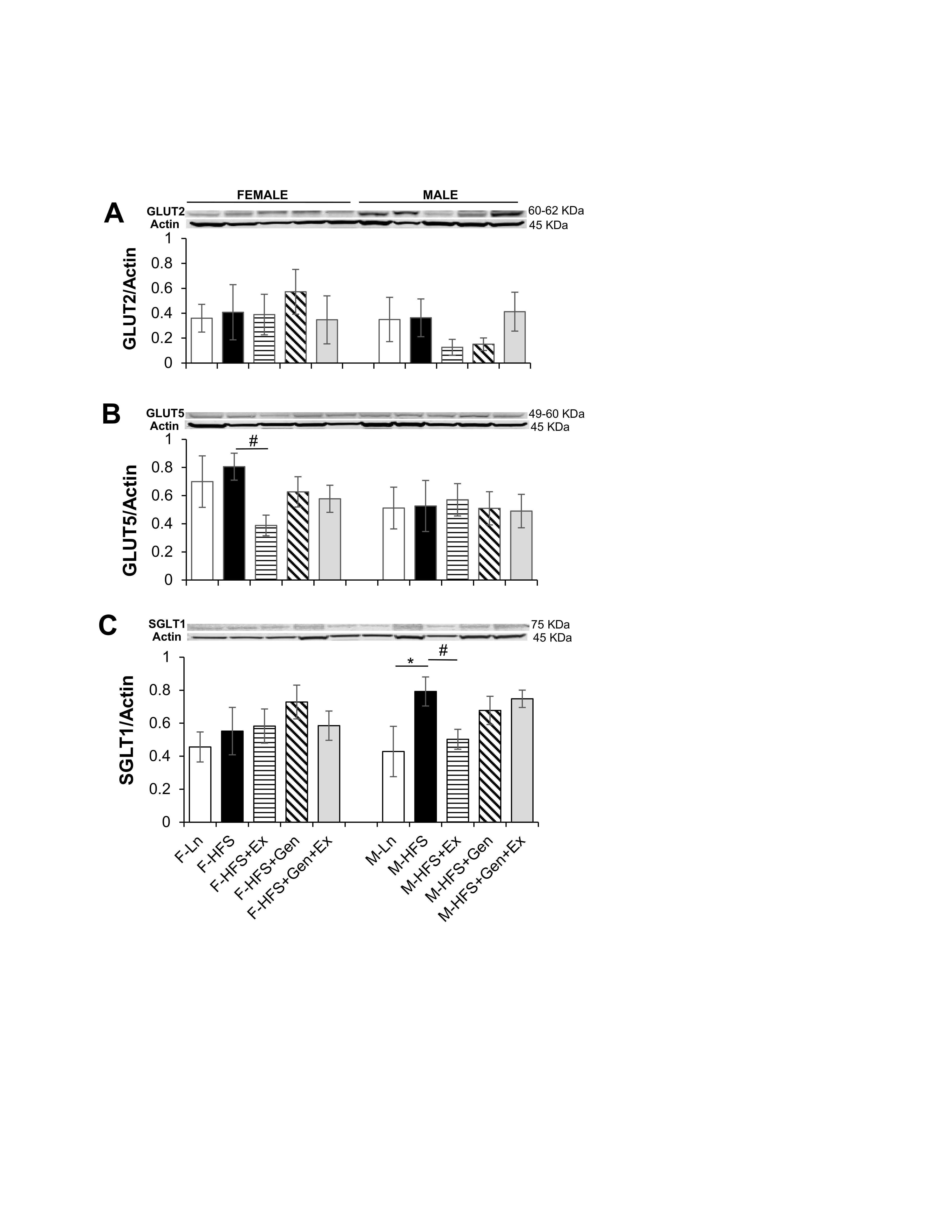
Fig. 4: Effect of high fat/high sugar diet, genistein and exercise on total expression of GLUT2, GLUT5, SGLT1 and PEPT1 expression in jejunum. A. Typical western blot demonstrating GLUT2 expression (normalized to Actin). Average GLUT2/Actin ratio is shown for all diet groups (n = 6/group). B. Typical western blot demonstrating GLUT5 expression (normalized to Actin). Average GLUT5/Actin ratio is shown for all diet groups (n = 5-7/group). C. Typical western blot demonstrating SGLT1 expression (normalized to Actin). Average SGLT1/Actin ratio is shown for all diet groups (n = 5-9/group). D. Typical western blot demonstrating PEPT1 expression (normalized to Actin). Average PEPT1/Actin ratio is shown for all diet groups (n = 4-7/group). Note: Controls, standard diet (Ln, open bars), high fat high sugar diet (HFS, solid black bars), HFS + exercise (HFS+Ex, horizontal line bars), HFS + genistein (HFS+Gen, hashed bars), HFS + genistein + exercise (HFS+Gen+Ex, gray bars). Data are means ± SEM. * denotes P<0.05, statistical difference to lean controls, and # denotes P<0.05, statistical treatment effect.
Intestinal health and cellular senescence
Total protein expression of p53 and pH2AX was evaluated to assess potential cellular damage and senescence. The induction of senescence by HFS, evidenced by increased expression of p53 in females (0.71 ± 0.19, n = 4, P <0.05) compared to lean controls (0.10 ± 0.05, n = 5, Fig. 5A ) was unchanged by treatments. In females, HFS-induced overexpression of p53 was accompanied by significant DNA damage as evidenced by a concomitant increase in pH2AX expression (0.72 ± 0.18, n = 4, P <0.05) compared to lean controls (0.31 ± 0.03, n = 6), which was significantly mitigated by Gen+Ex (0.31 ± 0.09, n = 8, P <0.05, Fig. 5B ). There were no changes in p53 or pH2AX expression in any of the groups of male mice. These data suggest that HFS diet may disrupt overall intestinal health and cellular senescence in females, but not males. Of note, these assessments are made in jejunum lysates as described in the methods section, and thus as a limitation for the study, do not assess specific cell types that are potentially targeted by HFS diet.
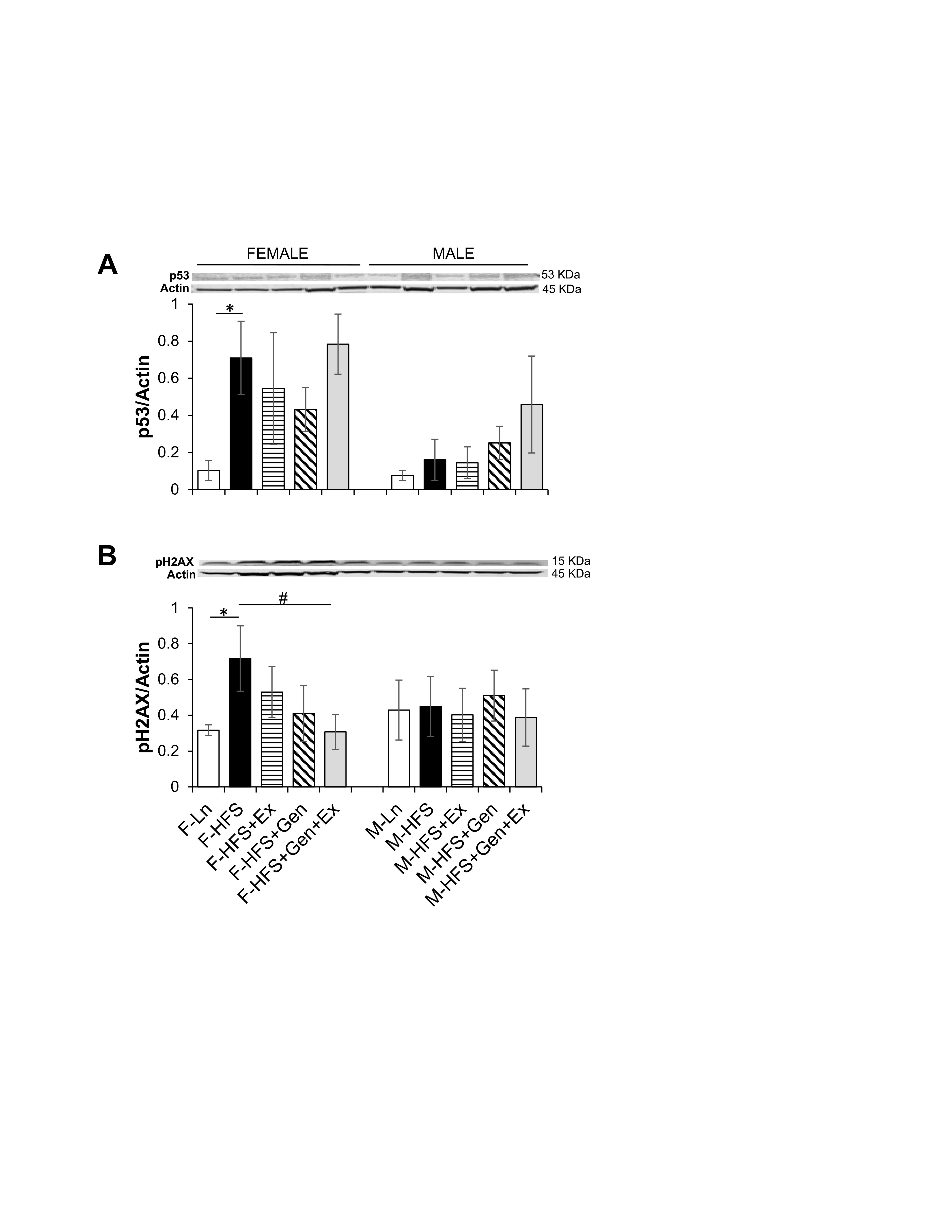
Fig. 5: Effect of high fat/high sugar diet, genistein and exercise on total expression of p53 and pH2AX proteins in jejunum. A. Typical western blot demonstrating p53 expression (normalized to Actin). Average p53/Actin ratio is shown for all diet groups (n = 5-6/group). B. Typical western blot demonstrating pH2AX expression (normalized to Actin). Average pH2AX/Actin ratio is shown for all diet groups (n = 4-8/group). Note: Controls, standard diet (Ln, open bars), high fat high sugar diet (HFS, solid black bars), HFS + exercise (HFS+Ex, horizontal line bars), HFS + genistein (HFS+Gen, hashed bars), HFS + genistein + exercise (HFS+Gen+Ex, gray bars). Data are means ± SEM. * denotes P < 0.05, statistical difference to lean controls, and # denotes P < 0.05, statistical treatment effect.
Jejunum Morphology
Modifications in either jejunum crypt or villi dimensions could alter the secretory and absorptive capacities respectively, while modifications in wall thickness could potentially alter motility. For example, a decreased crypt depth, could provide less available secretory epithelial cells and an increased villi length could provide a greater surface area for absorption within jejunum (or vice versa). Therefore, we determined whether HFS altered the morphology of the jejunum using H & E staining and analyzing for crypt depth, villi length and wall thickness. Villi length was increased 1.19-fold by HFS feeding in females compared to lean controls and there were no changes in villi length in males (Fig. 6A ). There were no changes in crypt depth in females and males (Fig. 6B ). Wall thickness was significantly reduced by HFS-diet in males (28.19 ± 1.85 µm, n = 5, P<0.05) compared to leans (37.40 ± 2.44 µm, n = 5) and this was mitigated by Ex (36.54 ± 0.84 µm, n = 6, Fig. 6C ). There were no changes in wall thickness between the HFS and lean female groups. These data suggest that structural changes in crypt dimension are not likely responsible for the reduced basal Isc noted in HFS-fed female and male mice.
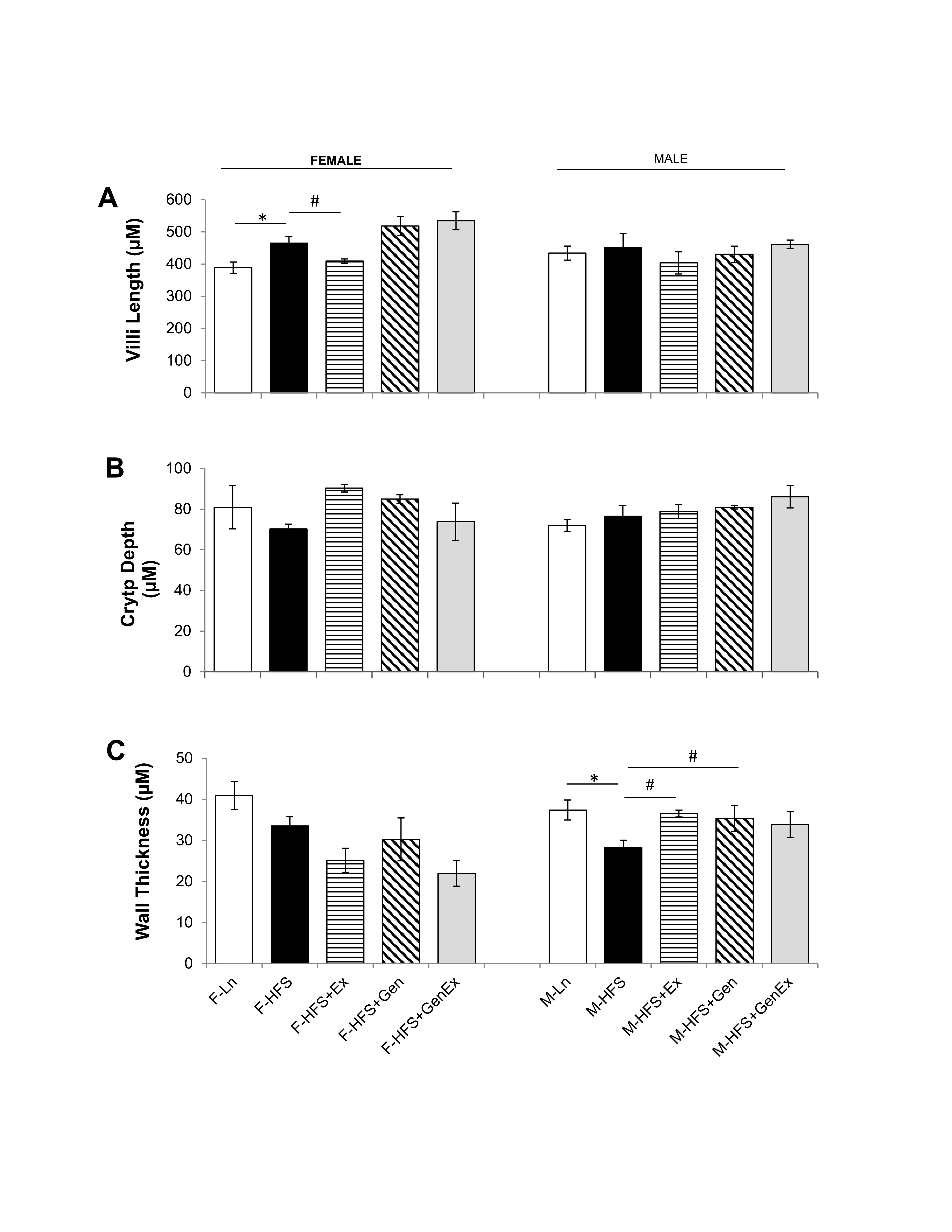
Fig. 6: Effect of high fat/high sugar diet, genistein and exercise on jejunum morphology. A. Average villi length in micrometers (n=4-6/group). B. Average crypt depth in micrometers (n=4-6/group). C. Average wall thickness (n=4-6/group). Note: Controls, standard diet (Ln, open bars), high fat high sugar diet (HFS, solid black bars), HFS + exercise (HFS+Ex, horizontal line bars), HFS + genistein (HFS+Gen, hashed bars), HFS + genistein + exercise (HFS+Gen+Ex, gray bars). Data are means ± SEM. * denotes P < 0.05, statistical difference to lean controls, and # denotes P < 0.05, statistical treatment effect.
Weight gain and Serum Profile
Changes in weight gain over the study duration are shown in Table 1 (females) and Table 2 (males). Female mice fed HFS exhibited a significant increase in weight gain during the study compared to lean, and those in the HFS+Gen+Ex group presented with significantly less weight gain compared to HFS alone (Table 1 ). Male mice fed HFS also exhibited a significant increase in weight gain compared to leans during the study and those in the HFS+Gen and HFS+Gen+Ex groups presented with significantly less weight gain compared to HFS alone (Table 2 ). In addition, in both male and female mice, serum glucose and insulin levels are suggestive of a diabetic state induced by HFS diet, i.e. hyperglycemia and hyperinsulinemia (Table 1 and 2 ). Both the hyperglycemia and hyperinsulinemia were mitigated by HFS+Gen+Ex in both females and males. Serum cytokine levels determined using a Milliplex assay are shown. In female mice, there were no changes in serum cytokine levels (Table 1 ). In male mice, the following serum cytokine levels were significantly increased by HFS consumption: TNF-alpha, IL-12, LIX, KC, MCP1 (Table 2 ). Treatment effects were variable; exercise significantly prevented the changes in serum MCP-1 and TNF-alpha levels; genistein prevented the changes in KC and TNF-alpha levels, and genistein+exercise combined prevented the changes in TNF-alpha, and MCP-1. In addition, we utilized an ELISA assay to quantify serum levels of fatty acid binding protein 1, FABP1, known to bind and transport cholesterol and fatty acids (Table 1 and 2 ). Interestingly, FABP1 was determined to be significantly increased by HFS diet in females only and exercise prevented this increase (no changes between groups in males). These FABP1 data suggest that males and females respond to the increase in caloric load differently.
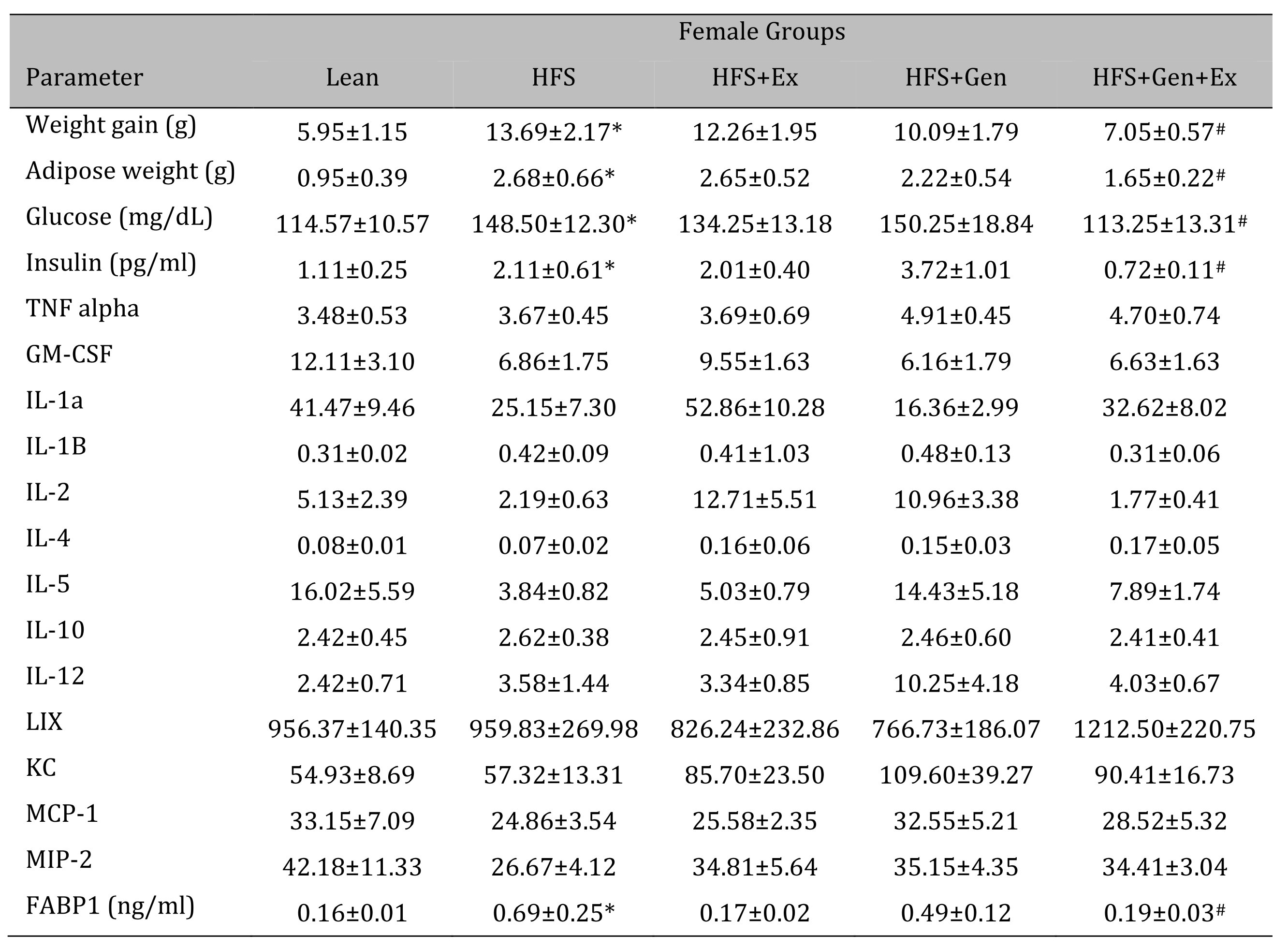
Table 1: The effects of exercise training, genistein, and combined exercise and genistein on weight gain and serum glucose, insulin, FABP1 and cytokine levels in female mice fed a high fat high sugar (HFS) diet. Values are reported as mean ± SEM for 8-10 mice/group for physical characteristics and for 5-10 mice/group for serum measures after 12 weeks treatment. All values for cytokines are in pg/ml. * indicates significant difference from lean control, P < 0.05, # indicates significant difference from HFHS control (treatment effect). Abbreviations: LIX, lipopolysaccharide-induced; KC, chemokine; IL, interleukin; MCP, monocyte chemoattractant protein; GM-CSF, granulocyte-macrophage colony-stimulating factor; MIP, macrophage inflammatory protein; TNFα, tumor necrosis factor alpha, FABP1, fatty acid binding protein-1
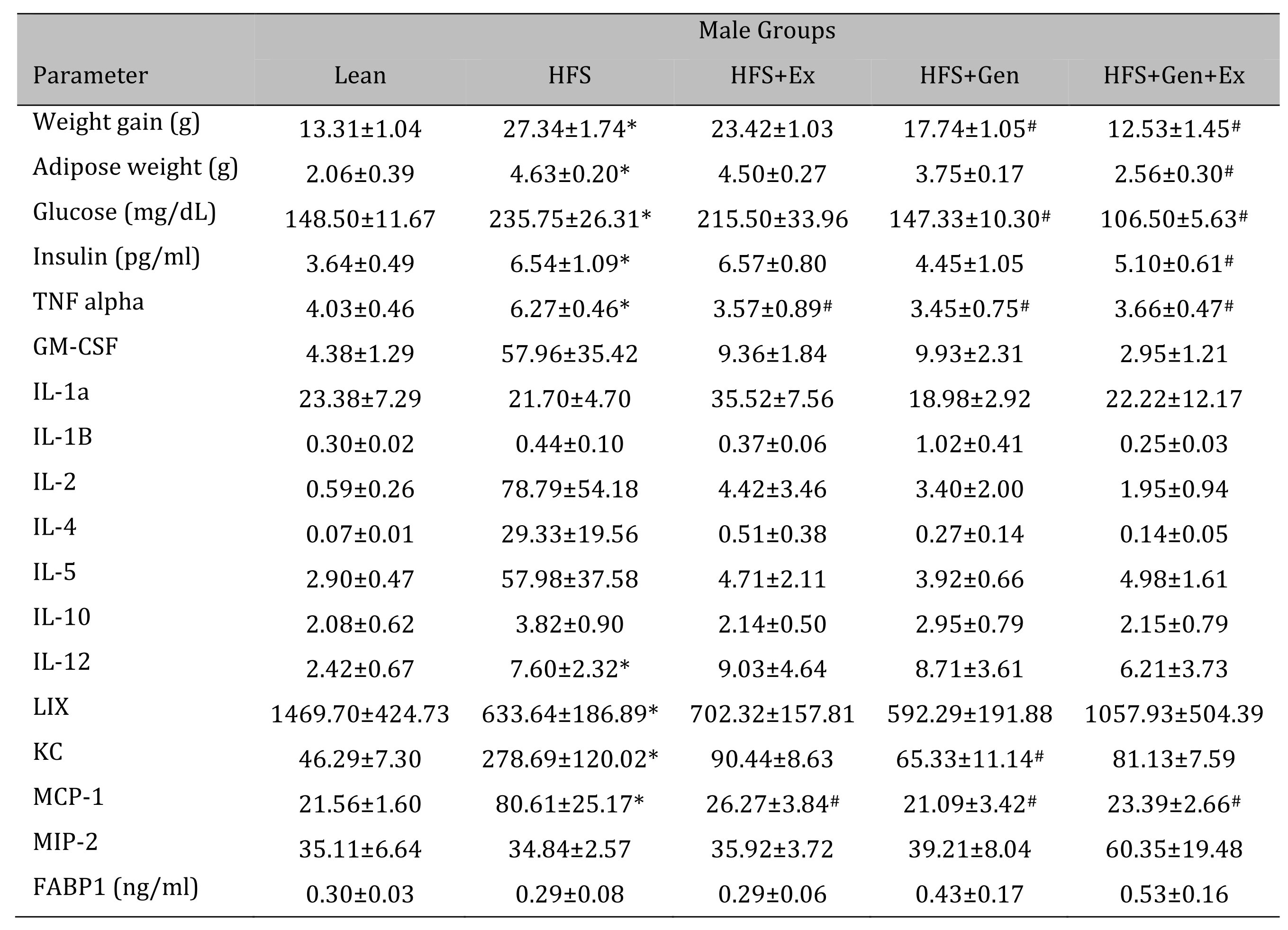
Table 2: The effects of exercise training, genistein, and combined exercise and genistein on weight gain and serum glucose, insulin, FABP1 and cytokine levels in male mice fed a high fat high sugar (HFS) diet. Values are reported as mean ± SEM for 8-10 mice/group for physical characteristics and for 5-10 mice/group for serum measures after 12 weeks treatment. All values for cytokines are in pg/ml. * indicates significant difference from lean control, P < 0.05, # indicates significant difference from HFHS control, P < 0.05. Abbreviations: LIX, lipopolysaccharide-induced; KC, chemokine; IL, interleukin; MCP, monocyte chemoattractant protein; GM-CSF, granulocyte-macrophage colony-stimulating factor; MIP, macrophage inflammatory protein; TNFα, tumor necrosis factor alpha, FABP1, fatty acid binding protein-1
Discussion
Western HFS diet induces diabetic obesity [24], however, the effects of western diet on small intestinal function remains an understudied area. Diabetes has been shown to elicit clinical gastrointestinal disturbances such as slowing of gastrointestinal transit [25], and gastroparesis [26]. Various murine models have been used to mimic the diabetic gastroparesis state, ob/ob mice (aged 6-15 weeks), and mice fed high fat diet (from age 14-33 weeks) [25, 27-30].
A main finding of the current study showed that there were significant deficits in HFS-fed mouse jejunum, the major site for fluid secretion in the small intestine. This is consistent with our previously published evidence indicating decreased secretory function in the ob/ob diabetic model [11, 23]. Specifically, in this western-diet fed model, we note significant reductions in: (1) small intestinal basal Isc in HFS mice compared to leans, (2) small intestinal forskolin-stimulated Isc in HFS mice compared to leans, and (3) bumetanide-sensitive Isc in HFS mice compared to leans. We note these significant changes are evident in both female and male mice fed HFS.
Small intestinal secretion requires appropriate function of key transporters on the apical and basolateral membrane of the enterocytes: Cl- enters the epithelial cells via the basolateral Na+/K+/2Cl- co-transporter, the Na+/K+-ATPase maintains Na+ and K+ concentration gradients across the membrane (the sodium gradient also drives glucose absorption), K+ is recycled across the basolateral membrane via clotrimazole-sensitive basolateral KCa channels [31-33], consequently maintaining a driving force for Cl- exit across the apical membrane, via calcium-activated and CFTR chloride channels [34-36]. Our data suggests that consumption of HFS diet resulted in reduced basal Isc and likely forskolin-stimulated Isc due to sex-dependent changes in key transporter expression: in female mice there was significant loss of CLC2 and CFTR protein expression and in male mice there was significant loss of Na+/K+-ATPase and Kca protein expression. A significant reduction in bumetanide-sensitive Isc was associated with reduced expression of Na+/K+/2Cl- co-transporter protein in both female and male HFS-fed mice.
An interesting relationship between expression of both Na+/K+-ATPase and SGLT1 has been shown previously by Serhan et al. [37], demonstrating that insulin increased glucose uptake with decreased Na+/K+-ATPase expression, and increased expression of SGLT1 in Caco-2 cells and rat jejunum. Our data is somewhat consistent with that of Serhan et al. [37], at least in males fed HFS and accompanied by an increase in serum insulin levels (Table 2), in that SGLT-1 expression was increased by HFS and Na+/K+-ATPase expression decreased with HFS. This finding in the HFS fed males was also prevented through exercise, reflected in a decrease in SGLT-1 and a concurrent increase in Na+/K+-ATPase expression, however, unlike Serhan’ study the exercise-induced effects were not associated with a concomitant decrease in insulin levels. These differences are likely attributed to dissimilarities in our respective studies, i.e., varied exercise intensity used and murine model.
Intestinal inflammation has been shown to influence ionic transport, via changes in expression/function or modification of the signaling pathways, of sodium channels, CFTR, Na+/K+-ATPase and NKCC1 [38, 39]. In experimental colitis, reduced colonic Isc has been shown to be a consequence of decreased chloride/bicarbonate secretion [39, 40]. Furthermore, increased levels of TNF-α or IFN-γ have been shown to have an inverse relationship with expression of CFTR protein [41, 42]. Since diabetes is associated with inflammation, this corresponds with the decrease in jejunum CFTR expression in HFS-fed female mice, and loss of NKCC1 expression in both male and female HFS-fed mice.
Exercise mitigated some of the HFS-induced decrease in basal Isc and, in males, there was an exercise-associated increase in Na+/K+-ATPase expression thus increasing the basal Isc towards that noted in lean controls. Interestingly, while exercise improved basal Isc in female HFS-fed mice, it was not associated with any modification in transporter protein expression. Furthermore, genistein significantly increased ClC2 expression in females without changing basal Isc. This suggests that ClC2 expression was not the major mechanism mediating basal Isc in female mice.
Genistein and insulin have been shown to synergistically stimulate insertion of NKCC1 into renal epithelial cells [43], however, in our studies, we did not note any treatment induced changes in NKCC1 expression. This could be attributed to a variety of differences in model systems and treatment dose/duration etc. For example, the presence of leptin in our model, as opposed to the ob/ob mice - a model used in previous studies, may have its own effect on Isc levels. It has been shown that leptin has a time dependent positive effect on Isc levels, resulting in an increase over time [44]. It has also been shown that leptin itself can act as a key mediator in gut inflammation, [45] and that a deficiency in this hormone may lead to a mouse model that is more resistant to developing specific forms of gut inflammation. This can contribute to varying levels of inflammatory markers and other cascading effects between diabetic murine models.
During inflammatory conditions, the endogenous signaling molecule adenosine (acting via the A2b receptor, A2bR) is known to be upregulated. Several studies have reported the involvement of A2BR in the pathophysiological mechanisms underlying several disorders relating to intestinal inflammation (mediated via an increase in TNFα) in colitis, obesity, and diabetes [6, 46-53]. Our data, demonstrating that A2BR expression was significantly increased by HFS in both females and males, is in accord with those above-mentioned studies. Interestingly, we note that both Gen and Gen+Ex mitigated this effect in females (not males).
In the current study, both sexes significantly increased weight gain over the 12-week HFS diet. While females increased weight by 13.69 ± 2.31 g (n = 9), males increased weight by 27.34 ± 1.73 g (n = 9); demonstrating 2 times more weight gain than their female equivalents. This suggests that the increase in weight gain with HFS feeding was sex dependent. The association between weight gain and increased inflammation is evident in our study across all male treatment groups. Reflected in measures of TNFα and MCP-1, as body weight increased with HFS feeding, we observed a correlative rise in inflammation markers. As body weight decreased with some treatments, the levels of inflammation also decreased. This is consistent with findings from other studies that showed there are clear associations between adiposity in mice and adipose tissue macrophage accumulation [54].
Theoretically, reduced crypt depth, could result in less secretory epithelial cells, and could explain in part the reduced basal Isc in HFS-fed mice. However, crypt depth was comparable in females and males fed HFS-fed compared to leans, suggesting that modifications in basal Isc were not a consequence of crypt structural changes.
Our study also clearly demonstrated variation in sex specific responses to treatment and pathology. Understanding sex-dependent differences has been an increasing area of focus and this has been supported throughout an emerging literature in the field, yet a clear need for further research remains. Gandhi et al. [55] showed sex differences in sugar uptake in mice across various organs and, while their study did not specifically show this in the small intestines, the impact of sex was well supported. Other investigations have shown markedly different sexually dimorphic results in weight gain, small intestinal transit, and serotonin dynamics in response to a high-fat diet. France et al. demonstrated that serotonin uptake was fluoxetine insensitive only in male mice fed a HFD [56], demonstrating that the effects of obesity were sex dependent and not simply a direct result of the HFD alone. Finally, Habib et al. [57] identified differences in weight gain across male and female mice fed a HFD. While both sexes gained weight in response to HFD (also demonstrated in our study), this was further exacerbated by Fabp6 deficiency and lead to further weight gain in males only [57]. This suggests not only that there are sex specific responses to HFD, but also that weight gain was not directly associated with fat absorption in the gut. This strongly supports the influence of secondary pathways in sex specific responses to HFD induced diabetes and obesity. The overall effects of HFS on female and male mice are summarized in Table 3 : the treatments we tested have a more robust mitigation in males versus females.
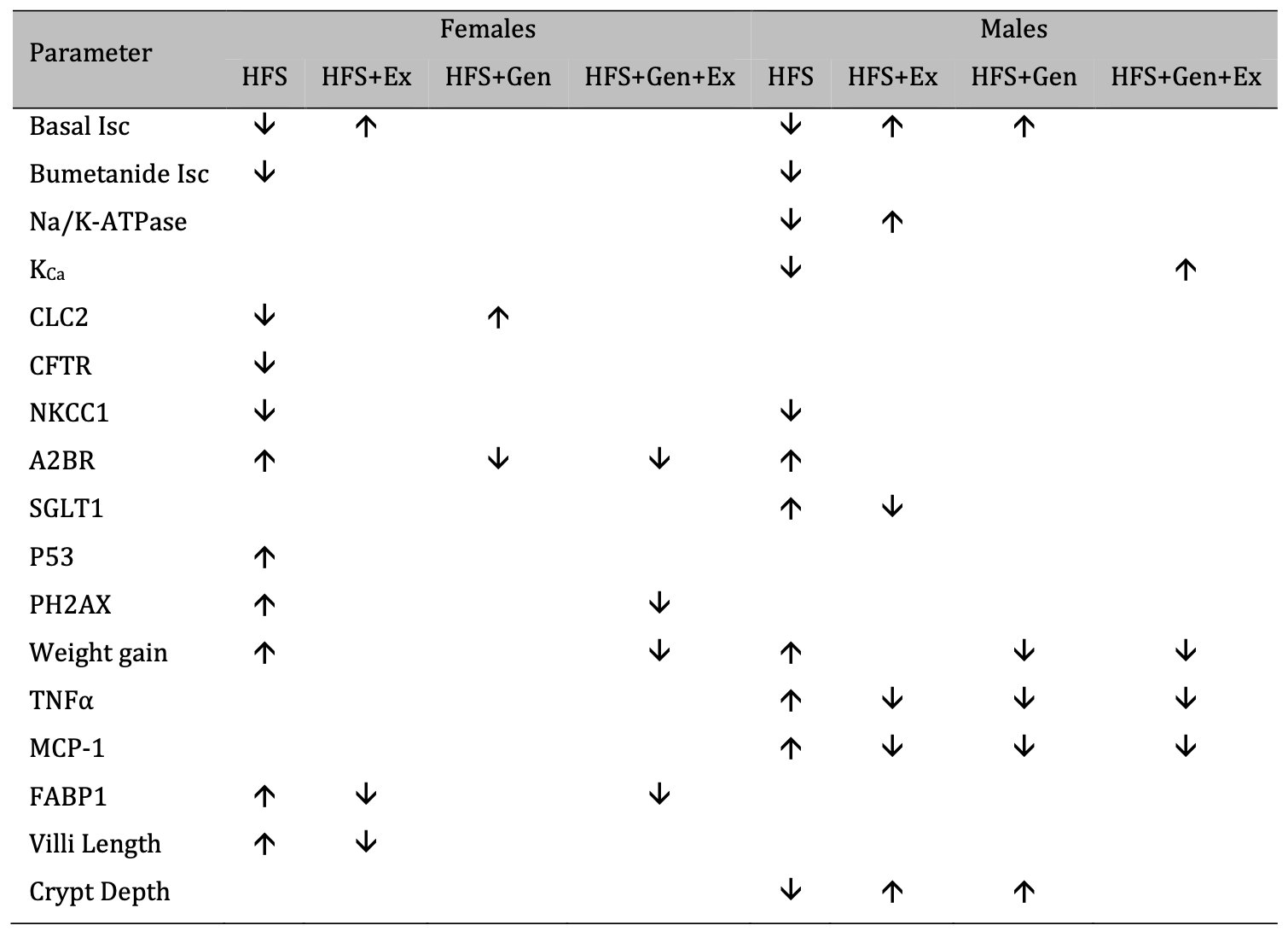
Table 3: A summary of the effects of exercise training, genistein, and combined exercise and genistein on mice fed a high fat high sugar (HFS) diet
Conclusion
Our data suggests that the reduced basal jejunal Isc in HFS mice is attributed to sex-dependent mechanisms and while exercise mitigated this in part, it’s mechanism of action was unclear. This work contributes significantly towards the current limited understanding of small intestinal function in diabetes, and obesity induced by consumption of western diets. Moreover, there are complex sex-dependent mechanism(s) of action of HFS on small intestinal physiology. Improved understanding of western diet induced intestinal dysfunctions may allow for the development of novel drug targets to treat gastrointestinal disturbances in obesity and diabetes.
Abbreviations
SGLT1 (sodium-dependent glucose transporter 1); GLUT2 (and GLUT5, glucose transporter protein 2 and 5); CFTR (cystic fibrosis transmembrane conductance regulatory chloride channel); NKCC1 (sodium/potassium/2 chloride-1 co-transporter); Na+/K+-ATPase (sodium/potassium-ATPase); KCa (calcium-activated potassium channel); A2BR (adenosine A2B receptor); CREB (cAMP response binding element); GAPDH (glyceraldehyde-3-phosphate dehydrogenase); H (& E, hematoxylin and eosin); FABP1 (fatty acid binding protein 1);
Acknowledgements
Brendan Van Iten (data collection), John Ahlert (data collection), Sarah Osborne (data collection and manuscript writing) and Chaheyla St Aubin (data collection) were each supported by the Midwestern University AZCOM KAS Summer Fellowship Program. Emma Smith (data collection) was supported by the College of Veterinary Medicine Summer Research Fellowship. Chaheyla St Aubin was supported by the Biomedical Sciences Program. All other authors K.M., M.R., A.K., K.S., B.L., K.M., S.A. all contributed towards data collection. LA conceptualized the project, created figures, contributed to manuscript writing and garnered funding. We thank Mr. Tatum Banayat for helpful technical assistance. All authors read and agreed to the published version of the manuscript.
Funding
This work was supported by Midwestern University Intramural funds (to L.A.), and a Midwestern Arizona Alzheimer’s Association Consortium grant (to L.A.), and Diabetes Action and Research Education Foundation (to L.A.).
Statement of Ethics/Ethical Approval
Animal experiments were in accordance with established guidelines, and all protocols were approved by the Midwestern University Institutional Animal Care and Use Committee (MWU-IACUC: AZ#2880). The study was conducted according to the guidelines of the Declaration of Helsinki.
Disclosure Statement
The authors have no disclosures and no conflicts of interests.
References
| 1 | Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J: Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health 2020;10:107-111.
https://doi.org/10.2991/jegh.k.191028.001 |
| 2 | Le Pluart D, Sabate J-M, Bouchoucha M, Hercberg S, Benamouzig R, Julia C: Functional gastrointestinal disorders in 35, 447 adults and their association with body mass index. Aliment Pharmacol Ther 2015;41:758-767.
https://doi.org/10.1111/apt.13143 |
| 3 | vd Baan-Slootweg OH, Liem O, Bekkali N, van Aalderen WMC, Pels Rijcken TH, Di Lorenzo C, Benninga MA: Constipation and colonic transit times in children with morbid obesity. J Pediatr Gastroenterol Nutr 2011;52:442-445.
https://doi.org/10.1097/MPG.0b013e3181ef8e3c |
| 4 | Hijo AHT, Coutinho CP, Alba-Loureiro TC, Leite JSM, Bargi-Souza P, Goulat-Silva F: High fat diet modulates the protein content of nutrient transporters in the small intestine of mice: possible involvement of PKA and PKC activity. Heliyon 2019;5:e02611.
https://doi.org/10.1016/j.heliyon.2019.e02611 |
| 5 | Bertrand RL, Senadheera S, Markus I, Liu L, Howitt L, Chen H, Murphy TV, Sandow SL, Bertrand PP: A Western diet increases serotonin availability in rat small intestine. Endocrinology 2011;152:36-47.
https://doi.org/10.1210/en.2010-0377 |
| 6 | Antonioli L, Pellegrini C, Fornai M, Tirotta E, Gentile D, Benvenuti L, Giron MC, Caputi V, Marsilio I, Orso G, et al: Colonic motor dysfunctions in a mouse model of high-fat diet-induced obesity: an involvement of A2B adenosine receptors. Purinergic Signal 2017;13:497-510.
https://doi.org/10.1007/s11302-017-9577-0 |
| 7 | Araujo JR, Tomas J, Brenner C, Sansonetti PJ: Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie 2017;141:97-106.
https://doi.org/10.1016/j.biochi.2017.05.019 |
| 8 | Murphy PA: Phytoestrogen content of processed soybean products. Food Technol 1982;36:60-64.
|
| 9 | Al-Nakkash L, Clarke LL, Rottinghaus GE, Chen YJ, Cooper K, Rubin LJ: Dietary genistein stimulates anion secretion across female murine intestine. J Nutr 2006; 136:2785-2790.
https://doi.org/10.1093/jn/136.11.2785 |
| 10 | Xu X, Wang H, Murphy P, Cook L, Hendrich S: Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr 1994;124:825-832.
https://doi.org/10.1093/jn/124.6.825 |
| 11 | Catmull S, Masood F, Schacht S, Dolan R, Stegman D, Leung L, Al-Nakkash L: Dietary genistein rescues reduced basal chloride secretion in diabetic jejunum via sex-dependent mechanisms. Cellular Physiology and Biochemistry 2016;40:335-346.
https://doi.org/10.1159/000452549 |
| 12 | Edwards KH, Ahuja KD, Watson G, Dowling C, Musgrave H, Reyes J, Cherry J, Kitic CM: The influence of exercise intensity and exercise mode on gastrointestinal damage. Appl Physiol Nutr Metab 2021;46:1105-1110.
https://doi.org/10.1139/apnm-2020-0883 |
| 13 | Yu T, Cai F, Jiang R: Effects of early bedside cycle exercise on gastrointestinal function in intesnive care unit patients receiving mechanical ventilation. Frontiers in Medicine 2022;9:823067.
https://doi.org/10.3389/fmed.2022.823067 |
| 14 | Veras K, Lucena CF, Goedcke J, Evangelista FS, Carpinelli A, de Oliveira Carvalho CR: Moderate exercise training combined with a high-fat and sucrose diet protects pancreatic islet function in male C57BL/6J mice. Front Endocrinol 2022;13.
https://doi.org/10.3389/fendo.2022.881236 |
| 15 | Wang J, Zhang Q, Xia J, Sun H: Moderate treadmill exercise modulates gut microbiota and improves intestinal barrier in high-fat-diet-induced obese mice via the AMPK/CDX2 signaling pathway. Diabetes Metab Syndr Obes: Targets and Therapy 2022;15:209-223.
https://doi.org/10.2147/DMSO.S346007 |
| 16 | Yu C, Liu S, Niu Y, Fu L: Exercise protects intestinal epithelial barrier from high fat diet-induced permeabilization through SESN2/AMPKa1/HIF-1a signaling. J Nutr Biochem 2022;107:109059.
https://doi.org/10.1016/j.jnutbio.2022.109059 |
| 17 | Sennott J, Morrissey J, Standley PR, Broderick TL: Treadmill exercise training fails to reverse defects in glucose. insulin and muscle GLUT4 content in the db/db mouse model of diabetes. Pathophysiology 2008;15:173-179.
https://doi.org/10.1016/j.pathophys.2008.06.001 |
| 18 | Leung L, Bhakta A, Cotangco K, Al-Nakkash L: Genistein stimulates jejunum chloride secretion via an Akt-mediated pathway in intact female mice. Cellular Physiology and Biochemistry 2015;35:1317-1325.
https://doi.org/10.1159/000373953 |
| 19 | Clarke LL, Harline MC: CFTR is required for cAMP inhibition of intestinal Na+ absorption in a cystic fibrosis mouse model. Am J Physiol 1996;270:G259-G267.
https://doi.org/10.1152/ajpgi.1996.270.2.G259 |
| 20 | Sheldon RJ, Malarchik ME, Fox DA, Burks TF, Porreca F: Pharmacological characterization of neural mechanisms regulating mucosal ion transport in mouse jejunum. J Pharmacol Exp Ther 1989;249:572-582.
|
| 21 | Clarke LL, Grubb BR, Gabriel SE, Smithies O, Coller BH, Boucher RC: Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science 1992;257:1125-1128.
https://doi.org/10.1126/science.257.5073.1125 |
| 22 | Lord R, Fairbourn N, Mylavarapu C, Dbeis A, Bowman T, Chandrashekar A, Banayat T, Hodges CA, Al-Nakkash L: Consuming genistein improves survival rates in the absence of laxative in deltaF508-CF female mice Nutrients 2018;10:1-12.
https://doi.org/10.20944/preprints201808.0084.v1 |
| 23 | Leung L, Kang J, Rayyan E, Bhakta A, Barrett B, Larsen D, Jelinek R, Willey J, Cochran S, Broderick TL, Al-Nakkash L: Decreased basal chloride secretion and altered CFTR, villin and GLUT5 protein expression in jejunum from ob/ob mice. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2014;7:1-10.
|
| 24 | St Aubin CR, Fisher AL, Hernandez JA, Broderick TL, Al-Nakkash L: Mitigation of MAFLD in High Fat-High Sucrose-Fructose Fed Mice by a Combination of Genistein Consumption and Exercise Training. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2022;15:2157-2172.
https://doi.org/10.2147/DMSO.S358256 |
| 25 | El-Salhy M: Gastrointestinal transit in an animal model of human diabetes type 2: relationship to gut neuroendocrine peptide contents. Ups J Med Sci 2002;107:101-110.
https://doi.org/10.3109/2000-1967-133 |
| 26 | Asakawa A, Inui A, Ueno N, Makino S, Uemoto M, Fujuno MA, Kasuga M: Ob/ob mice as a model of delayed gastric emptying. J Diabetes Complications 2003; 17:27-28.
https://doi.org/10.1016/S1056-8727(02)00198-8 |
| 27 | Aviello G, Matias I, Capasso R, Petrosino S, Borelli F, Orlando P, Romano B, Capasso F, Di Marzo V, Izzo AA: Inhibitory effect of the anorexic compound oleoylethanolamide on gastric emptying in control and overweight mice. J Mol Med 2008;86:413-422.
https://doi.org/10.1007/s00109-008-0305-7 |
| 28 | Bhetwal BP, An C, Baker SA, Lyon KL, Perrino BA: Impaired contractile responses and latered protein expression and phosphorylation of Ca2+ sensitization proteins in gastric antrum smooth muscles from ob/ob mice. J Muscle Res Cell Motil 2013;34:137-149.
https://doi.org/10.1007/s10974-013-9341-1 |
| 29 | Verhulst PJ, Lintermans A, Janssen S, Loeckx D, Himmelreich U, Buyse J, Tack J, Depoortere I: GPR39, a receptor of the ghrelin receptor family, plays a role in the regulation of glucose homeostasis in a mouse model of early onset diet-induced obesity. J Neuroendocrinology 2011;23:490-500.
https://doi.org/10.1111/j.1365-2826.2011.02132.x |
| 30 | El-Salhy M, Spangeus A: Gastric emptying in animal models of human diabetes: correlation to blood glucose level and gut neuroendocrine peptide content. Ups J Med Sci 2002;107:89-99.
https://doi.org/10.3109/2000-1967-132 |
| 31 | Devor DC, Singh AK, Gerlach AC, Frizzell RA, Bridges RJ: Inhibition of intestinal Cl- secretion by clotrimazole: direct effect on basolateral membrane K+ channels. Am J Physiol 1997;273:C531-C540.
https://doi.org/10.1152/ajpcell.1997.273.2.C531 |
| 32 | Hamilton KL, Meads L, Butt AG: 1-EBIO stimulates Cl- scretion by activating a basolateral K+ channel in the mouse jejunum. Pflugers Arch 1999;439:158-166.
https://doi.org/10.1007/s004249900137 |
| 33 | Al-Nakkash L, Batia L, Bhakta M, Peterson A, Hale N, Skinner R, Sears S, Jensen J: Stimulation of murine intestinal secretion by daily genistein injections: gender-dependent differences. Cellular Physiology and Biochemistry 2011;28:239-250.
https://doi.org/10.1159/000331736 |
| 34 | Anderson MP, Welsh MJ: Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci USA 1991;88:6003-6007.
https://doi.org/10.1073/pnas.88.14.6003 |
| 35 | Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A, Boucher RC: Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(-/-) mice. Proc Natl Acad Sci USA 1994;91:479-483.
https://doi.org/10.1073/pnas.91.2.479 |
| 36 | Grubb BR: Ion transport across the normal and CF neonatal murine intestine. Am J Physiol 1999;277:G167-G174.
https://doi.org/10.1152/ajpgi.1999.277.1.G167 |
| 37 | Serhan MF, Kreydiyyeh SI: Insulin down-regulates the Na+/K+ ATPase in enterocytes but increases intestinal glucose absorption. General and Comparative Endocrinology 2010;167:228-233.
https://doi.org/10.1016/j.ygcen.2010.03.010 |
| 38 | Uribe JM, McCole DF, Barrett KE: Interferon-g activates EGF receptor and increases TGF-alpha in T84 cells: implications for chloride secretion. Am J Physiol 2002;283:G923-931.
https://doi.org/10.1152/ajpgi.00237.2002 |
| 39 | Martinez-Augustin O, Romero-Calvo I, Suarez MD, Zarzuelo A, Sanchez de Medina F: Moelcular bases of impaired water and ion movements in inflammatory bowel diseases. Inflamm Bowel Dis 2009;15:114-127.
https://doi.org/10.1002/ibd.20579 |
| 40 | Bell CJ, Gall DG, Wallace JL: Disruption of colonic electrolyte transport in experiemntal colitis. Am J Physiol 1995;268:G622-630.
https://doi.org/10.1152/ajpgi.1995.268.4.G622 |
| 41 | Nakamura H, Yoshimura K, Bajocchi G, Trapnell BC, Pavirani A, Crystal RG: Tumor necrosis factor modulation of expression of the cystic fibrosis transmembrane conductance regulator gene. FEBS Letters 1992;314:366-370.
https://doi.org/10.1016/0014-5793(92)81507-I |
| 42 | Besancon F, Przewlocki G, Baro I, Hongre A-S, Escande D, Edelman A: Interferon-gamma down regulates CFTR gene expression in epithelial cells. Am J Physiol 1994;267:C1398-1404.
https://doi.org/10.1152/ajpcell.1994.267.5.C1398 |
| 43 | Ueda-Nishimura T, Niisato N, Miyazaki H, Naito Y, Yoshida N, Yoshikawa T, Nishino H, Marunaka Y: Synergic action of insulin and genistein on Na+/K+/2Cl- cotransporter in renal epithelium. Biochem Biophys Res Commun 2005; 332:1042-1052.
https://doi.org/10.1016/j.bbrc.2005.05.046 |
| 44 | Hoda MR, Scharl M, Keely SJ, McCole DF, Barrett KE: Apical leptin induces chloride secretion by intesinal epithelial cells and in a rat model of acute chemotherapy-induced colitis. Am J Physiol 2010;298:G714-G721.
https://doi.org/10.1152/ajpgi.00320.2009 |
| 45 | Siegmund B, Lehr HA, Fantuzzi G: Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology 2002;122:2011-2025 doi: 2010.1053/gast.2002.33631.
https://doi.org/10.1053/gast.2002.33631 |
| 46 | Hasko G, Csoka B, Nemeth ZH, Vizi ES, Pacher P: A2B adenosine receptors in immunity and inflammation. Trends Immunol 2009;30:263-270.
https://doi.org/10.1016/j.it.2009.04.001 |
| 47 | Frick J-S, MacManus CF, Scully M, Glover LE, Eltzchig HK, Colgan SP: Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol 2009;182:4957-4964.
https://doi.org/10.4049/jimmunol.0801324 |
| 48 | Kolachala V, Asamoah V, Wang L, Srinivasan S, Merlin D, Sitaraman SV: Interferon-gamma down regulates adenosine 2b receptor-mediated signaling and short cicuit current in the intestinal epithelia by inhibiting the expression of adenylate cyclase. J Biol Chem 2005;280:4048-4057.
https://doi.org/10.1074/jbc.M409577200 |
| 49 | Kolachala V, Asamoah V, Wang L, Obertone TS, Ziegler TR, Merlin D, Sitaraman SV: TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signalling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci 2005;62:2647-2657.
https://doi.org/10.1007/s00018-005-5328-4 |
| 50 | Kolachala VL, Vijay-Kumar M, Dalmasso G, Yang D, Linden J, Wang L, Gewirtz A, Ravid K, Merlin D, Sitaraman SV: A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterol 2008;135:861-870.
https://doi.org/10.1053/j.gastro.2008.05.049 |
| 51 | Csoka B, Koscso B, Toro G, Kokai E, Virag L, Nemeth ZH, Pacher P, Bai P, Hasko G: A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes 2014;63:850-866.
https://doi.org/10.2337/db13-0573 |
| 52 | Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, Pankow JS, Ravid K, Fredholm B, Hedrick CC, Rich SS, et al: Links between insulin resistance, adenosine A2B receptors and inflammatory markers in mice and humans. Diabetes 2011;60:669-679.
https://doi.org/10.2337/db10-1070 |
| 53 | Johnston-Cox H, Koupenova M, Yang D, Corkey B, Gokce N, Farb MG, LeBrasseur N, Ravid K: The A2b adenosine receptor modulates glucose homeostatsis and obesity. PLos ONE 2012;7:e40584.
https://doi.org/10.1371/journal.pone.0040584 |
| 54 | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796-1808.
https://doi.org/10.1172/JCI200319246 |
| 55 | Gandhi A, Tang R, Seo Y, Bhargava A: Organ-Specific Glucose Uptake: Does Sex Matter? Cells 2022;11:2217 doi: 2210.3390/cells11142217.
https://doi.org/10.3390/cells11142217 |
| 56 | France M, Skorich E, Kadrofske M, Swain GM, Galligan JJ: Sex-related differences in small intestinal transit and serotonin dynamics in high-fat-diet-induced obesity in mice. Exp Physiol 2016;101:81-99.
https://doi.org/10.1113/EP085427 |
| 57 | Habib SM, Zwicker BL, Wykes L, Agellon LB: Sexually dimorphic response of mice to the Western-style diet caused by deficiency of fatty acid binding protein 6 (Fabp6). Physiol Rep 2021;9:e14733.
https://doi.org/10.14814/phy2.14733 |