Original Article - DOI:10.33594/000000669
Accepted 4 November 2023 - Published online 15 November 2023
Do the Effects of Krebs Cycle Intermediates on Oxygen-Dependent Processes in Hypoxia Mediated by the Nitric Oxide System Have Reciprocal or Competitive Relationships?
Keywords
Abstract
Background/Aims:
Currently, it is proven that the cellular metabolism of nitric oxide is necessary to maintain optimal health and adaptation of the organism to the impact of various environmental factors. The aim of this work was to reveal the biological role of nitric oxide, its metabolic changes, and its mechanism of action in tissues under hypoxia, as well as the possibility of tissue metabolism correction through NO-dependent systems under the influence of Krebs cycle intermediates.Materials:
A systematic assessment of the effect of succinate (SC, 50 mg/kg b.w.) and α-ketoglutarate (KGL, 50 mg/kg b.w.) in the regulation of oxygen-dependent processes in rats (mitochondrial oxidative phosphorylation, microsomal oxidation, intensity of lipid peroxidation processes, and the state of the antioxidant defense system) depending on functional changes in nitric oxide production during hypoxia was evaluated. The state of the nitric oxide system was estimated spectrophotometrically by determination of the concentration of its stable nitrite anion metabolite (NO2–). The levels of catecholamines were estimated from the content of epinephrine and norepinephrine using the differentially fluorescent method. The activity of cytochrome P450-dependent aminopyrine-N-demethylase was determined with the Nash reagent.Results:
Tissue hypoxia and metabolic disorders caused by this condition through changes in the content of catecholamines (epinephrine, norepinephrine, dopamine, DOPA) as well as the cholinesterase-related system (acetylcholine content and acetylcholinesterase activity) were the studied experimental parameters under acute hypoxia (AH, 7% O2 in N2, 30 min). The activation of lipid peroxidation and oxidatively modified proteins and an increase in the epinephrine content in AH are associated with an increased role of SC and a decrease in KGL as substrates of oxidation in mitochondria. A more pronounced effect of exogenous KGL, compared to SC, on the content of nitrite anion as a stable metabolite of nitric oxide in the liver under acute hypoxia against the background of a decrease in the intensity of lipid peroxidation processes was revealed. The activation of SC-dependent mitochondrial oxidative processes caused by AH was found to decrease in animals after an intermittent hypoxia training (IHT) course. IHT (7% O2 in N2, 15-min, 5 times daily, 14 days) prevented the activation of oxidative stress in tissues and blood after the AH impact and increased the efficiency of energy-related reactions in the functioning of hepatic mitochondria through increased oxidation of KGL.Conclusion:
The studied effects of adaptation are mediated by an increase in the role of NO-dependent mechanisms, as assessed by changes in the pool of nitrates, nitrites, carbamides, and total polyamines.Highlights
- SC and KGL significantly affect the functional changes in the nitrite and nitrate components of the nitric oxide system;
- The effects of SC and KGL in acute and intermittent hypoxia are opposite by affecting the nitric oxide system;
- SC effect in the blood on the production of nitric oxide to be higher than that of KGL;
- KGL affects the nitric oxide system under acute hypoxia by increasing the efficacy of oxygen consumption;
- SC and KGL have a multifactorial effect on the content of nitrite and nitrate in the blood and liver.
Introduction
The current understanding of the physiological, therapeutic, and prophylactic effects of hypoxia is associated with the elucidation of the mechanisms of energy production, in which mitochondria play a leading role [1, 2]. However, microsomal oxidation and the level of antioxidant protection against free radical production are also important oxygen-dependent processes in various pathologies accompanied by hypoxic phenomena. It is suggested that approximately 80% of oxidative metabolism and nearly 50% of total drug elimination can be attributed to one or more of the various cytochrome P450 enzymes in this system [3]. The role of coordinated work of these three mechanisms of oxygen-dependent processes in pathology is often associated with the functioning of the Krebs cycle. This cycle seems to be a central point of cell metabolism or a central route for oxidative metabolism [4]. The role of the Krebs cycle is related to the production of NADH and FADH2, which fuel the mitochondrial electron transport chain that produces ATP [5, 6]. This cycle is also a reliable source of metabolic intermediates necessary for anabolic reactions [7]. Therefore, numerous literature data indicate that hypoxic-induced damage to cells and tissues, which occurs when the supply of oxygen to tissues and organs is inadequate, is considered a phased process of dysfunction of mitochondrial enzyme complexes [8]. The latter ultimately leads to the inhibition of aerobic energy synthesis, energy-dependent functions, and cell metabolism [9]. Therefore, in the cascade of metabolic disorders in hypoxia, pharmacological correction of energy supply processes with the introduction of natural non-toxic metabolites of the body is of particular importance [10]. Important mechanisms that connect the tricarboxylic acid (TCA) cycle or Krebs cycle and chromatin modifications are shown in the study conducted by Wellen et al. [11].
Bioactive metabolites in the organism include metabolites of the Krebs cycle, which have been actively studied over the past decade due to the wide range of bioregulatory effects, in particular in the case of significant extreme stress encountered by the organism during hypoxic processes. Since α-ketoglutarate (KGL) is a key modulator of the hypoxic response, its use is an effective therapeutic tool due to the involvement of hypoxia-inducible factor-1 (HIF-1) in these processes [12-15]. The finding that α-ketoglutarate has multiple functions in physiology, regulating epigenetic changes [16] with a concomitant increase in the α-ketoglutarate-to-succinate ratio, as shown by Carey et al. [17], may be associated with attractive therapeutic opportunities in treatment of diseases [18].
In addition to α-ketoglutarate, the literature also presents the effects of succinate, which is a metabolite of the Krebs cycle serving many intracellular and metabolic functions. Succinate (SC) is considered an oncometabolite that accumulates due to inactivating mutations in the succinate dehydrogenase (SDH) gene. It is regarded as an oncometabolite because SDH mutations are common in some cancers [19]. In addition, SC can inhibit pyruvate dehydrogenases (PHDs), promoting the accumulation of HIF-1α in the presence of oxygen; this phenomenon is known as pseudohypoxia [20]. Therefore, by modulating cellular processes, including metabolism and signaling, Krebs cycle intermediates are able to influence the processes of cancer development and progression [21, 22].
The conclusions regarding the mechanisms of adaptation of the human organism to the conditions of low partial pressure of oxygen in alveolar air can be divided into two components [23-25]. The first specific component of adaptation is the series of structural and functional changes aimed at improving the oxygen supply to tissues. The second nonspecific component of adaptation is the activation of cellular and humoral immunity mechanisms, the cytochrome P450 detoxification system, and antioxidant defense systems [26].
Violation of the mechanisms of energy support under hypoxia depends on the disorders of oxidative phosphorylation due to the disintegration of respiratory processes and oxidative phosphorylation, switching metabolism to the glycolysis path, which nevertheless does not provide sufficient energy production [27, 28]. These changes also determine the functionality of the Krebs cycle. Hypoxia can lead to regulatory reprogramming of the respiratory chain [29], in particular, to suppression of the electron transport function of mitochondrial enzyme complex I (NAD-dependent oxidation) and activation of mitochondrial complex II (FAD-dependent oxidation), as reported by Lukyanova et al. [8]. These effects correlate with the substrates of NAD-dependent (α-ketoglutarate) and FAD-dependent (succinate) mitochondrial respiratory chain function [29-31, 6]. In the context of acute oxygen deficiency, alternative ways of regulating oxygen-dependent processes associated with the activation of aminotransferase reactions for the formation of α-ketoglutarate and its rapid oxidation into succinate may be activated8. Such a strategy provides a rapid supply of high-energy substrates such as succinate for subsequent oxidation in the respiratory chain of mitochondria.
The N-demethylation reaction of aminopyrine (4-dimethylamino-2, 3-dimethyl-l-phenyl-3-pyrazolin-5-one) by microsomal enzymes has been clarified to be a monooxygenase-type reaction [32]. The hepatic microsomal monooxygenase system, containing cytochrome P450 as a terminal oxidase, is functional in the oxidative biotransformation of a wide variety of endogenous and exogenous substances [33, 34, 26, 3]. The physiological role of this enzyme system has been investigated in hypoxic conditions to clarify the physiological and toxicological relationships between cytochrome P450 and its catalytic activities during succinate and ketoglutarate treatment under acute hypoxia are not well represented in literature. Despite the large number of reports on animal liver microsomal activity, to our knowledge, there are no extensive studies of in vitro aminopyrine N-demethylation in animal liver microsomes in which comparative analysis of the impact of Krebs cycle intermediates under acute hypoxia was performed. Considering this fact, it is difficult to establish whether there are significant differences in this activity in comparison to different types of oxygen-dependent processes (mitochondrial oxidation, lipid peroxidation, antioxidant enzyme functioning).
The production of nitric oxide, which is an effective regulator of mitochondrial respiratory chain function, is no less important in hypoxia [35, 36]. It is known that, with participation of the enzyme NO-synthase (NOS), L-arginine is converted into citrulline with the release of NO [37]. Since this reaction is oxygen-dependent, the formation of NO under hypoxia is significantly reduced [38]. Arginine synthesis is carried out through the condensation of citrulline with aspartate and the subsequent formation of an intermediate product such as arginine-succinate. After breakdown, this substrate forms arginine and fumarate, which links the cycle of carbamide with the Krebs cycle [39]. As shown by recent data, the NO-dependent mechanisms in the pathogenesis of many diseases, i.e. heart failure and myocardial ischemia, are mediated by the involvement of mitochondrial functioning [40], mitochondrial NOS under chronic hypoxia and ischemia-reperfusion41, mitochondrial arginine metabolism [42], and efficient breast cancer therapy [43].
However, studies on the comparative analysis of the effect of the two substrates of the Krebs cycle, succinate and alpha-ketoglutarate, given their wide network of biochemical connections of metabolic pathways with the nitric oxide system, on the regulation of oxygen-dependent processes in mitochondria and redox chains in microsomes, taking into account oxidative stress and mediator balance, are not sufficiently represented in the literature. On the other hand, it is equally important to consider these interdependencies in conditions of acute hypoxia loads and the formation of long-term adaptive responses to these loads. A comparative analysis and comparison of the development of three oxygen-dependent processes simultaneously in two models of hypoxia exposure are of practical importance since the literature presents the exposure effects for each of these metabolic pathways separately. The significance of our study and the identification of existing gaps in knowledge regarding oxygen-induced processes under the influence of succinate and α-ketoglutarate (as substrates of the Krebs cycle) will help to reveal the causal relationships between independent and dependent variables caused by hypoxia of different genesis, as well as adaptation to it. We aimed to carry out the comparative study due to specific mechanisms underlying the protective effects of sodium alpha-ketoglutarate and sodium succinate on mitochondrial phosphorylation processes, microsomal oxidation by N-demethylation of aminopyrine, and oxidative stress biomarkers (lipid peroxidation, oxidatively modified proteins, activities of antioxidant enzymes) in hepatic tissue of Wistar rats under acute hypoxia (7% O2 in N2, 30 min) and intermittent hypoxia training (7% O2 in N2, 15 min, 5 times daily, 14 days).
Materials and Methods
Animals and experimental design
Animals.
The experiments were conducted in compliance with the Guidelines of the European Union Council, the current laws in Poland and Ukraine, and the recommendations of the Ethical Commission. They were approved by the Ethical Commission of T.G. Shevchenko National University “Chernihiv Collegium” (05/02/2019). The experiment was carried out in accordance with both the Directive 2010/63/EU on the protection of animals used for scientific purposes and with the Polish Act of 15 January 2015 on the protection of animals used for scientific or educational purposes (JL, February 26, 2015, Pos.266). To eliminate diurnal rhythm changes, all examinations started at 10 am and ended at 12 am.
Male white rats (180-220 g) were used in the current study. The rats were housed at a constant temperature of 21 ± 2oC. The animals had free access to feed and water throughout the experiments.
Experimental groups.
The rats were randomly assigned into ten groups. 1) An untreated control group (n = 6); 2-3) groups that received intraperitoneally 50 mg of sodium succinate per kg body weight (n = 6) or 50 mg of sodium α-ketoglutarate per kg body weight (n = 6); the time of action was 30 min; 4) an acute hypoxia group (7% O2 in N2, 30 min). Before the 30-min acute hypoxia exposure, the rats were injected with 1 mL of saline (n = 6). The rats of this group were kept in a special chamber ventilated with a mixture of air with 7% oxygen in nitrogen for 30 minutes. An absorbent was placed in the chamber to absorb carbon dioxide and water vapor. After the 30-min acute hypoxia exposure, the animals were decapitated; 5) a group that received intraperitoneally 50 mg of sodium succinate per kg body weight (n = 6) before acute hypoxia exposure (7% O2 in N2, 30 min). After the 30-min acute hypoxia exposure, the animals were decapitated; 6) a group that received intraperitoneally 50 mg of sodium α-ketoglutarate per kg body weight (n = 6) before acute hypoxia exposure (7% O2 in N2, 30 min). After the 30-min acute hypoxia exposure, the animals were decapitated. The last four groups of animals (n = 6 in each group) were used in the experiment after 14 days of the course of intermittent hypoxic training (IHT). For this purpose, every day the animals were placed in a chamber, which was alternately ventilated in 15-min intervals with a gas mixture of 10% oxygen in nitrogen and room air (21% O2). The number of such cycles was 5 per day. Every day, 30 min before the hypoxic training, these rats were parenterally injected with 1 ml of saline. The day after the last training session, all animals were tested by acute hypoxia exposure (7% oxygen in nitrogen, 30 min); immediately after the test, the animals were animals were killed by intraperitoneal injection of a lethal dose of sodium pentobarbital (Morbital; Biowet, Pulawy; 200 mg/kg b.w.).
Sample collection.
Blood samples were collected after sacrificing the rats into plain tubes (to obtain serum), heparinized tubes (to obtain heparinized plasma), and tubes with EDTA 10% as an anticoagulant (to obtain EDTA plasma). The samples were centrifuged at 3, 000 rpm for 10 min to obtain plasma. After the separation of plasma, the buffy coat with white cells was removed. Erythrocytes were washed three times with saline by centrifugation at 3, 000 rpm for 10 min at 4°C. Blood samples, plasma, and erythrocytes were used for the study immediately.
Livers were removed from rats after decapitation. One animal was used for each mitochondrial preparation. The isolated liver was excised, weighed, and washed in ice-cold buffer containing 120 mM KCl, 2 mM K2CO3, 10 mM HEPES, and 1 mM EGTA. The reaction was adjusted to pH 7.2 with KOH. The minced tissue was rinsed with cold isolation buffer to eliminate the blood and then transferred to a glass Potter-Elvehjem homogenizing vessel with a motor-driven Teflon pestle on ice. The suspension was then centrifuged for 3 min at 600 g at 0oC. The hepatic tissue suspension was used for biochemical assays. Mitochondria from the liver were isolated with the method of differential centrifugation as in work [44] Kondrashova and Doliba (1989). The resulting supernatants were re-centrifuged at 10, 000 g for 20 min. Finally, the mitochondria were re-suspended in the isolation buffer. The mitochondrial suspension (4-6 mg of protein/mL) was kept on ice before the experiment.
For the preparation of microsomes, a part of the liver was immediately chilled in ice-cold 1.15% KCl-buffer (pH 7.4). Tissue manipulations were performed in a cold room to maintain the liver temperatures from 0 to 4oC. The frozen tissue was homogenized in ice-cold homogenization buffer (0.1M Tris-HCl, 1.15% KCl, pH 7.4) in a glass Potter-Elvehjem-type homogenizer with a Teflon pestle. Microsomes were prepared as described by Wisniewski et al. [45]. The homogenized liver was centrifuged at 16, 800 g for 20 min in a temperature range from 0 to 4oC. The supernatant was centrifuged at 15, 000 g for 60 min, and the cytosol was kept and frozen at -80oC. The microsomal pellet was re-suspended in 15 ml of 0.4 M sucrose and 77 mM sodium pyrophosphate (pH 7.5) and re-centrifuged as before. The final pellet was re-suspended in 150 mM KCl to yield about 30 mg protein per mL. The protein concentration was determined with the Bradford assay using bovine serum albumin as a standard [46].
Oxygraphic measurement of mitochondrial respiration.
Oxygen uptake was measured using a Clark-type oxygen probe immersed in a magnetically stirred sample chamber (1 ml) in a water bath. The rate of oxygen uptake was expressed as ng at O per min per mg of mitochondrial protein. The mitochondria were added to the respiration chamber containing 120 mM KCl, 2 mM K2CO3, 2 mM KH2PO4, and 10 mM HEPES. Potassium hydroxide (1.0 N) was used to adjust the pH of the medium to 7.20 at 26oC. A-ketoglutarate (1 mM of the final concentration), succinate (0.35 mM), glutamate (3 mM), malate (2.5 mM), and pyruvate (3 mM) were used as oxidative substrates. ADP at a concentration of 0.2 mM was administered as a phosphate acceptor. Inhibitory analysis with the use of rotenone (10 µM), an inhibitor of complex I activity in the mitochondrial electron transport chain, malonate (2 mM), a competitive inhibitor of succinate oxidation by complex II, and 1 mM aminooxiacetate, an inhibitor of the transamination process, was carried out to estimate the role of al
NADH- and FADH-generated substrates in the processes of mitochondrial oxidation.
The following mitochondrial oxygen consumption parameters were analyzed: state 2 (oxygen consumption before the addition of ADP), state 3 (oxygen consumption stimulated by ADP), and state 4 (oxygen consumption after cessation of ADP phosphorylation). The ADP-to-oxygen ratio (ADP/O) was calculated as the ratio of nanomoles of added ADP per nanogram atoms of oxygen utilized during state 3. The respiratory control ratio described by Chance (ratio of state 3 to state 4) and the ADP/O ratio (ratio between the nanomoles of phosphorylated ADP and the nanomoles of oxygen consumed during state 3) were determined. The method proposed by Chance and Williams [47-49] was used for analysis of the respiratory control ratio (RCR), calculation of the ADP/O ratio, and determination of the phosphorylation rate Vph in μmol ADP per min per mg protein. The respiratory control ratio defined by Chance was calculated as the ratio of state 3 to state 4 respiration rates. The oxygen consumption was determined with the use of oxidation substrates (succinate or α-ketoglutarate) or inhibitors of the mitochondrial respiratory chain in the presence (state 3) or the absence (state 4) of ADP (phosphate acceptor) and next recorded as nanogram oxygen atoms per minute per milligram of mitochondrial protein. The protein concentration was determined with the Bradford assay46 using bovine serum albumin as a standard.
Biochemical assays
Measurement of aminopyrine-N-demethylase activity
The activity of cytochrome P450-dependent aminopyrine-N-demethylase was determined using the method described by authors [50]. The method is based on the definition of the formaldehyde reaction with the Nash reagent [51]. The reaction proceeds in the presence of cytochrome P450, NADPH, and oxygen. The incubation media contained: 3 mM NADPH(H), 0.1 M Tris-hydrochloride, 0.25 M Tris, 8 mM aminopyrine, and 5 mM magnesium chloride. The color of the resulting complex was measured at 412 nm. The enzymatic activity of aminopyrine-N-demethylase was calculated from the calibration curve, which was based on a standard formaldehyde solution. The hepatic microsomal activity was determined from the above results and expressed in nmoles of formaldehyde produced per min per mg protein of microsomes.
Nitric oxide system assays.
The state of the nitric oxide system was estimated spectrophotometrically by determination of the concentration of its stable nitrite anion metabolite (NO2–) using the method proposed by Green et al. [52] and expressed in pmol per mg protein. The content NO3– was determined with the method described by authors [53]. This method is based on the reaction of the nitrate ion with brucine sulfate in an H2SO4 solution at a temperature of 100°C. The color of the resulting complex is measured at 410 nm. Temperature control of the color reaction is extremely critical. The level NO3– was expressed in nmol per mg protein. The level of carbamides was assessed in a reaction with diacethylmonooxime [54], while the content of total polyamines was determined spectrophotometrically from the amount of putrescine in a reaction with 2, 4-dinitrofluorobenzene [55].
2-Thiobarbituric acid reactive substances (TBARS) assay.
TBARS were measured with the method developed by author [56]. The TBARS level was expressed in nmol of malonic dialdehyde (MDA) per mL or nmol of MDA per mg protein.
Protein carbonyl derivative assay.
The level of oxidatively modified proteins (OMP) was estimated by the reaction of the resultant carbonyl derivatives of amino acids with 2, 4-dinitrophenyl hydrazine (DNFH) as described by Levine et al. [57] with modification introduced by author [58]. Carbonyl groups were determined spectrophotometrically at 370 nm (aldehyde derivatives, AD OMP) and 430 nm (ketonic derivatives, KD OMP) and expressed in nmol per mL or nmol per mg of protein.
Superoxide dismutase activity assay.
Superoxide dismutase (SOD) activity in the supernatant was determined according to method [59]. SOD activity was assessed by its ability to dismutate superoxide produced during quercetin auto-oxidation in an alkaline medium (pH 10.0). The activity was expressed in units of SOD per mL or units of SOD per mg of protein.
Catalase activity assay.
Catalase (CAT) activity was determined by measuring the decrease in the H2O2 level in the reaction mixture using the method proposed by author [60]. One unit of CAT activity was defined as the amount of the enzyme required for the decomposition of 1 μmol H2O2 per min per mL or mg of protein.
Glutathione reductase activity assay.
Glutathione reductase (GR) activity in the samples was measured with the method described by Glatzle and co-workers [61] with some modifications. The GR activity was expressed as nmol of NADPH2 per min per mL or mg of protein.
Glutathione peroxidase activity assay.
Glutathione peroxidase (GPx) activity was determined by detecting the nonenzymatic utilization of GSH (the reacting substrate) at an absorbance of 412 nm after incubation with 5, 5-dithiobis-2-nitrobenzoic acid (DTNB) with the method developed by Moin [62]. The GPx activity was expressed as nmol GSH per min per mL or mg of protein.
Alanine aminotransferase (AlAT) and aspartate aminotransferase (AsAT) activity assay.
AlAT and AsAT activity was analyzed spectrophotometrically using a standard enzymatic method described by author [63]. One unit of AsAT or AlAT activity was defined as the liberation of 1 μmol of pyruvate per min of incubation at 37°C per mL or mg protein.
Succinate dehydrogenase (SDH) activity assay.
SDH activity was analyzed spectrophotometrically with the method developed by [64]. One unit of SDH activity was defined as the amount of the enzyme required for the decomposition of 1 nmol succinic acid per min per mg of protein.
Level of cholinergic system activity.
Cholinergic system activity was estimated by the level of non-mediated acetylcholine (ACh) by adapting the technique described by author [65] and modified by Menshikov [66], and acetylcholinesterase activity (AChE) in blood was assessed by adapting the technique described by Ellman et al. [67]. Briefly, 0.2 mL of samples were added to a solution containing 1.0 mM acetylthiocholine (AtCh), 0.1 mM of Ellman’s reagent [5, 5-dithio-bis-(2-nitrobenzoic acid, DTNB], and 100 mM phosphate buffer (pH 8.0). Immediately before and after incubation for 30 min at 27°C, the absorbance was read on a spectrophotometer at 412 nm. The results were expressed as µmol of ACh per min per mL.
Catecholamine balance.
The levels of catecholamines were estimated from the content of epinephrine and norepinephrine using the differentially fluorescent method [68] with aluminum and potassium sulfate and by adapting the fluorometric hydroxyindole assay method described by authors [69] and based on iodine oxidation, alkaline rearrangement, and subsequent measurement of the fluorescence of the final solution at an acidic pH. The concentration of epinephrine and its precursor (dopamine, DOPA) was estimated using the method proposed by Jacobovitz and Richardson [70]. All samples were read on a Hitachi fluorescence spectrophotometer (Model 650-10M) with excitation slit 2 nm, emission slit 10 nm, and sensitivity 1. The fluorescence activation wavelengths were 380-480 nm for norepinephrine and 410-500 nm for epinephrine.
Statistical analysis.
The results were expressed as mean ± standard deviation (S.D.). All variables were tested for normal distribution using the Kolmogorov-Smirnov and Lilliefors tests (p > 0.05), and homogeneity of variance was checked using Levene’s test. The significance of differences in the level of enzymes and substrates between the control and examined groups was assessed using ANOVA, Student’s t-test, and Mann–Whitney U test. Differences were considered significant at p < 0.05. In addition, the relationships between the data of all individuals were evaluated using Spearman correlation analysis [71]. All statistical calculations were performed on separate data from each individual with STATISTICA 8.0 software (StatSoft Inc., Poland).
Results
Effect of succinate and α-ketoglutarate on mitochondrial respiration
The first step in our study consisted in a comparative analysis of the effect of two substrates of the Krebs cycle, succinate (SC) and ketoglutarate (KGL), on their oxidation in mitochondria induced by acute hypoxia (7% O2 in N2, 30 min). These data are shown in Table 1. When studying the state of energy supply under acute hypoxia, it should be noted that changes in the biochemical mechanisms of the impact of this extreme factor are associated with a significant increase in the ADP-stimulated respiration level when SC is mainly used as an oxidation substrate, compared to KGL. The percentage of the increase in the oxidation of SC is significant. with an increase in the coupling of respiration and phosphorylation (respiratory control by Chance) and the rate of phosphorylation of added ADP. However, the rate of oxygen consumption, as evidenced by the ADP/O value, decreased with a trend toward significance. A decrease in the oxygen concentration in inhaled air is not accompanied by significant suppression of the oxidation of NADH-dependent substrates, in particular KGL. Thus, under acute hypoxia, a predominant increase in the role of SC in total metabolic oxidation was shown. The effects of SC under acute hypoxia are not associated with the activation of respiration in the V3 state, but the efficiency of phosphorylation was increased significantly during oxidation with SC as a substrate. The КGL oxidation was accompanied by a significant decrease in the ADP/O level.
The effect of KGL on the functional state of mitochondria in the rats under acute hypoxia was associated with a decrease in the effects of oxidation of SC as a substrate and an increase in the respiratory coefficient by Chance and ADP/O. However, the rate of ADP phosphorylation in these conditions was low. The oxidation of endogenous KGL was accompanied by a decrease in phosphorylation respiration in state V3, phosphorylation efficiency, and its rate. These changes occurred while maintaining a high value of the coupling of respiration and phosphorylation processes, which exhibited a statistically higher level in the group of the acute hypoxia-exposed rats. Thus, the multidirectional changes in the oxidation of these two substrates are determined by reciprocal mechanisms of their influence, i.e. the introduction of SC under acute hypoxia reduces the oxidation of KGL, while the influence of KGL limits the effects of SC on the processes of energy supply in mitochondria. These effects can be elucidated in an inhibition analysis using malonate, a competitive inhibitor of SDH, in the oxidation of КGL. This allows elimination of the component that endogenous succinate contributes to the oxidation of КGL in the mitochondria when maximum involvement of energy production processes during acute oxygen deficiency is required to stabilize mitochondrial dysfunction (Table 1).
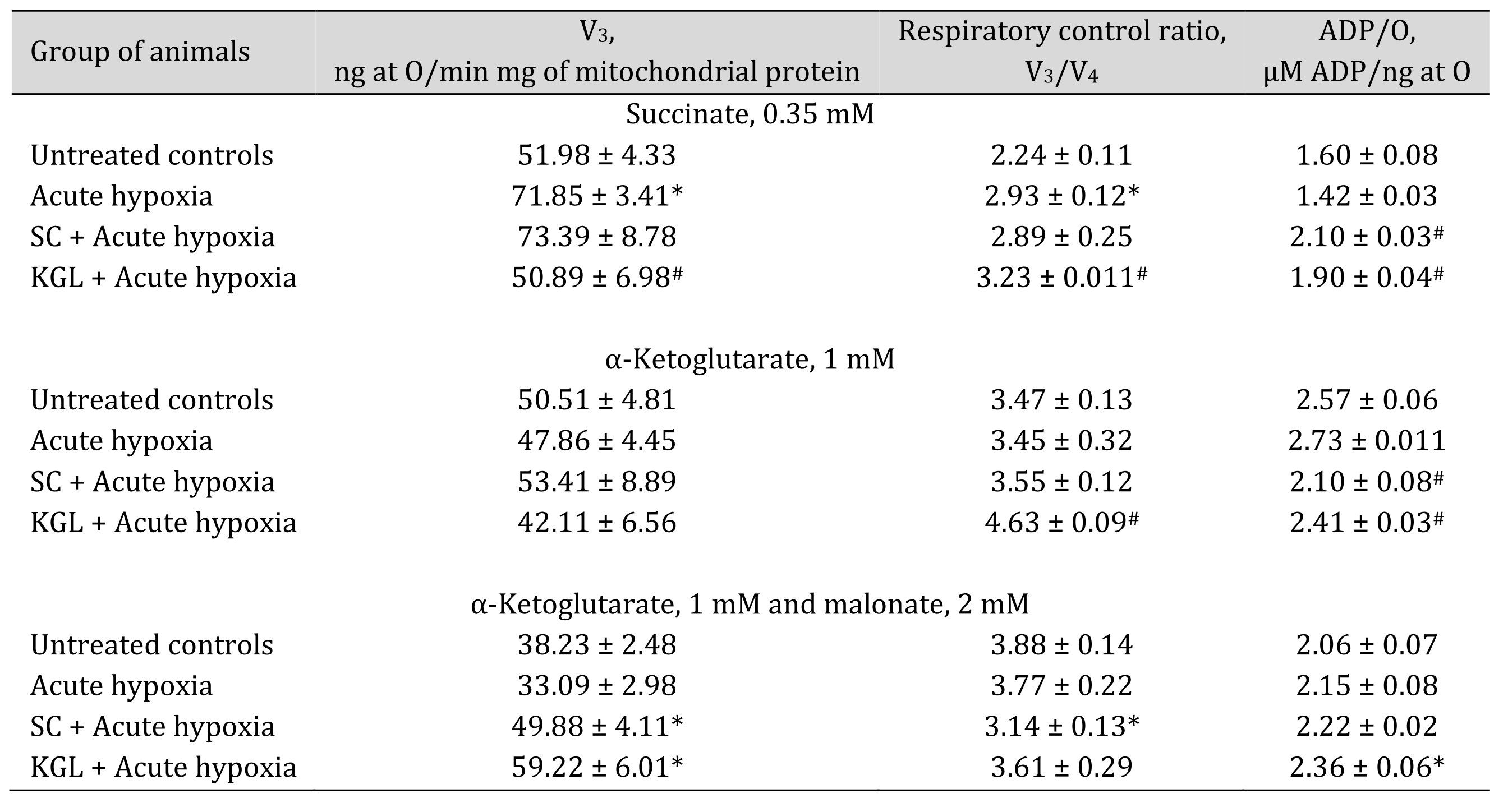
Table 1: Effect of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on mitochondrial respiration in the hepatic tissue of rats under acute hypoxia (AH, 7% O2 in N2, 30 min) in the presence of 0.35 mM succinate or 1 mM α-ketoglutarate as substrates of mitochondrial respiration as well as 2 mM malonate as an SDH inhibitor. Oxygen uptake by hepatic mitochondria was measured polygraphically in the presence of 0.35 mM succinate or 1 mM α-ketoglutarate as well as 2 mM malonate as an SDH inhibitor. ADP was administered at a concentration of 0.2 mM as a phosphate acceptor. * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p < 0.05) between the group exposed to acute hypoxia and groups treated with succinate or α-ketoglutarate under acute hypoxia
Effects of succinate and α-ketoglutarate on aminotransferase and succinate dehydrogenase activity
With the aim of verifying the hypothesis that aminotransferase reactions are involved in the effects of exogenous SC and KGL under acute hypoxia, we measured mitochondrial oxidation using glutamate and malate as substrates, which induce aminotransferase reactions, simultaneously with oxaloacetate, an inhibitor of processes of transamination (Table 2), and directly assessed the activities of alanine aminotransferase (AlAT) and aspartate aminotransferase (AsAT) (Table 3).
The use of glutamate and malate as substrates of oxidation in mitochondria caused activation of oxidative phosphorylation under the impact of both SC and КGL, which was shown in the mitochondrial respiration parameters. We obtained an increase in respiration during SC oxidation and a simultaneous significant decrease in the ADP/O value when the rats were administered SC before acute hypoxia, compared to values obtained in the rats after the acute hypoxia impact. In contrast, when the rats were administered КGL under acute hypoxia, a significant increase in the respiration values was observed for the same oxidation substrates with an increase in respiratory control, and the ADP/O values were low, relative to those obtained in the rats under acute hypoxia. Since the effects of glutamate and malate oxidation on the respiration rate, respiratory control, and ADP/O were significantly reduced by the administration of SC and КGL to the rats, it was concluded that this pathway is important for the effects of exogenous КGL. These results were also confirmed by the direct measurement of aminotransferase activity under acute hypoxia and the effects of the two aforementioned substances (Table 3).
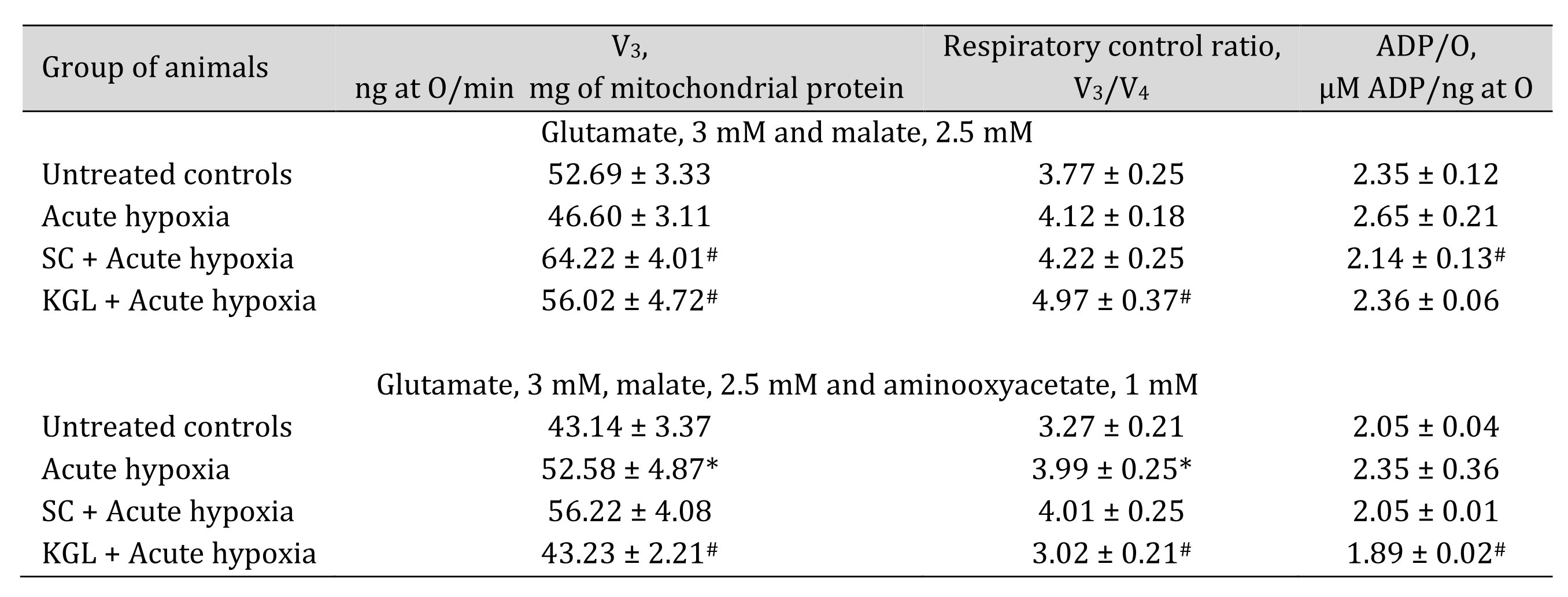
Table 2: Effect of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on mitochondrial respiration in the hepatic tissue of rats at acute hypoxia (AH, 7% O2 in N2, 30 min) in the presence of 3 mM glutamate and 2.5 mM malate as substrates of mitochondrial respiration, as well as 1 mM aminooxyacetate as an nonselective inhibitor of transaminases. Oxygen uptake by hepatic mitochondria was measured polygraphically in the presence of 3 mM glutamate and 2.5 mM malate as substrates as well as 1 mM aminooxyacetate as a nonselective inhibitor of transaminases. ADP was administered at a concentration of 0.2 mM as a phosphate acceptor. * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p < 0.05) between the group exposed to acute hypoxia and groups treated with succinate or α-ketoglutarate under acute hypoxia
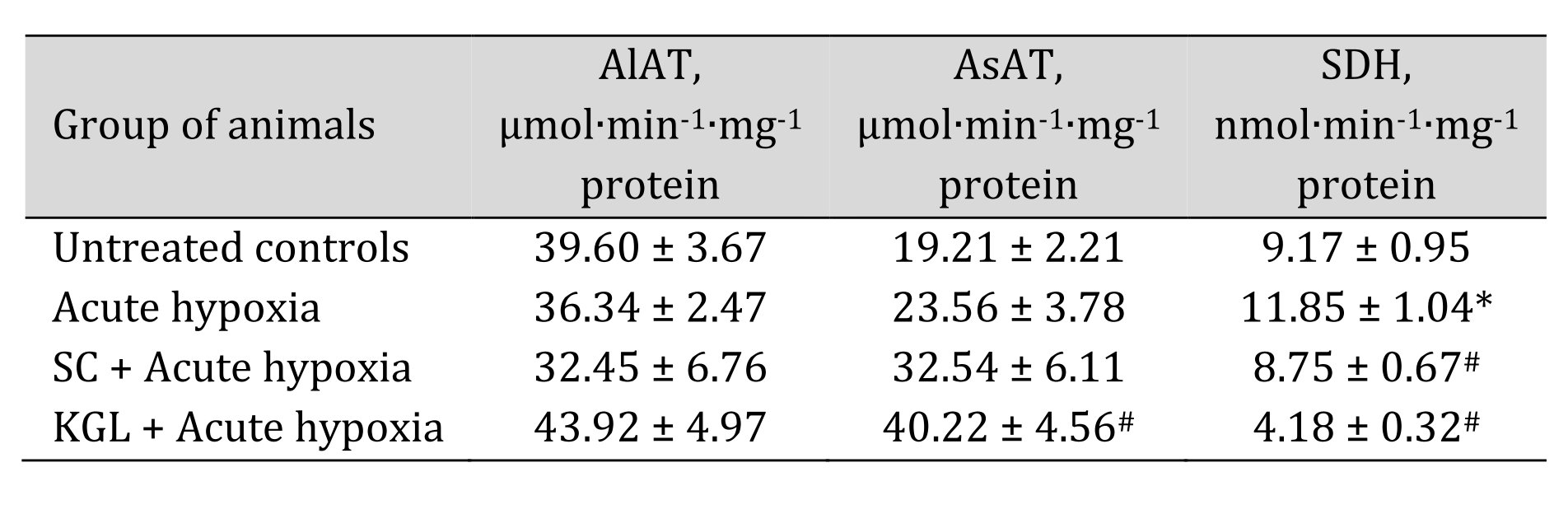
Table 3: Effect of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on activities of alanine- (AlAT) and aspartate (AsAT) aminotransferase (μmol∙min-1∙mg-1 protein) as well as succinate dehydrogenase (SDH, nmol∙min-1∙mg-1 protein) in the hepatic tissue of rats (n = 6) at acute hypoxia (7% O2 in N2, 30 min). * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p < 0.05) between the group exposed to acute hypoxia and groups treated with succinate or α-ketoglutarate under acute hypoxia
Aminopyridine-N-demethylase activity of the hepatic microsomal fraction
It is known that one of the metabolic pathways carried out by the cytochrome P450 system is the N-demethylation process, and aminopyrine is one of the most commonly used reagents to study this cellular pathway. The assessment of the N-demethylase activity of aminopyrine was the next stage of our research. It is shown in Fig. 1. The N-demethylase activity of aminopyrine was determined using hepatic microsomes prepared from the rats in various states (normoxia or hypoxia) at the SC and KGL impact. This method should be useful for clarifying the relationship between the microsomal monooxygenase system and physiologically or toxicologically induced alterations in the liver during the steep decline of oxygen. The enzyme activity of aminopyrine-N-demethylase in our studies, which may simulate microsomal oxidation states in hepatic tissue of rats, was significantly decreased after the acute hypoxia, while SC administered to the rats before this exposure statistically significantly increased the activity compared to the values obtained under acute hypoxia in the rats.
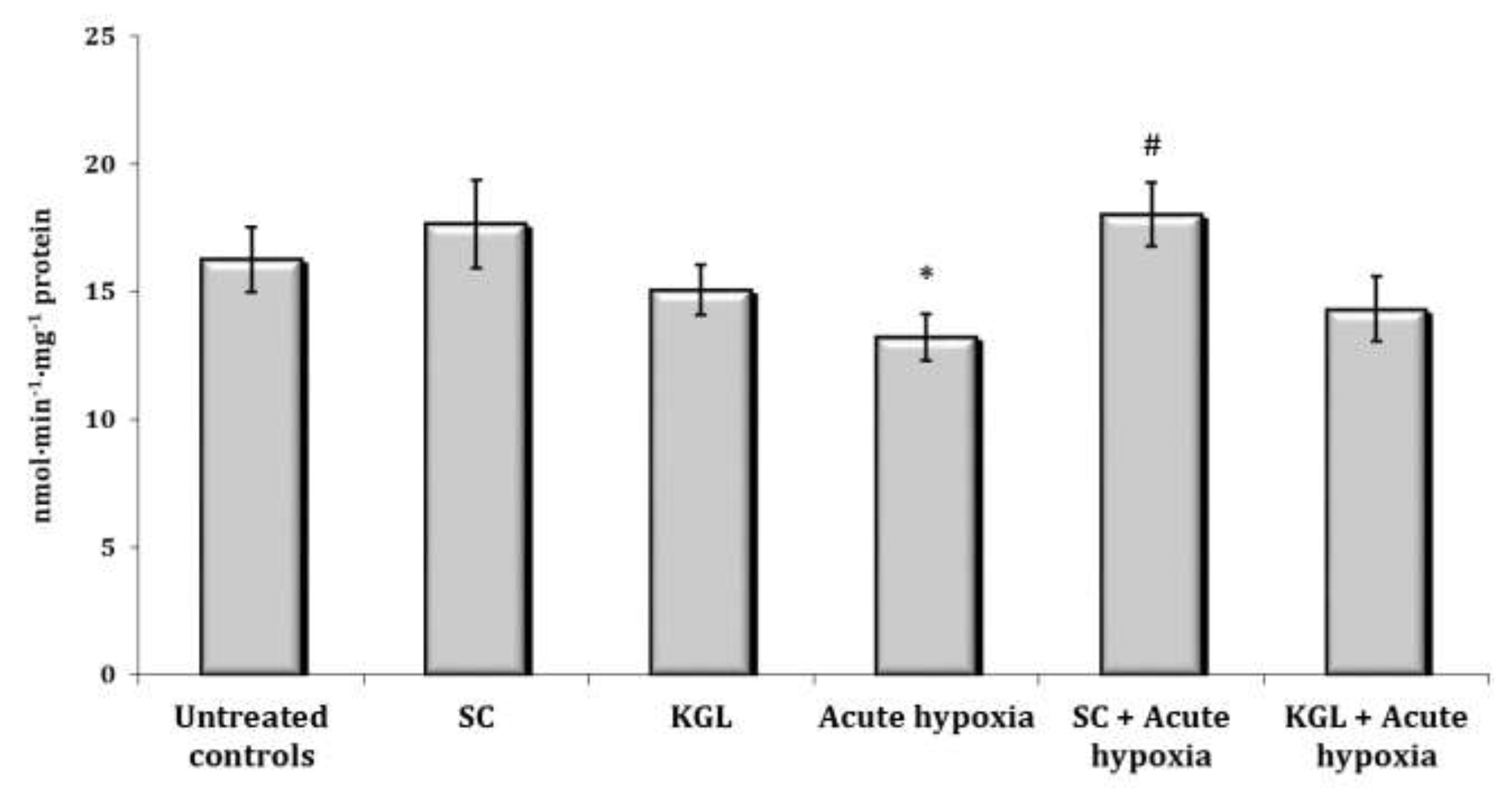
Fig. 1: Effects of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on aminopyrine-N-demethylase activity (nmol∙min-1∙mg-1 protein) in the liver of rats (n = 6) at acute hypoxia (7% O2 in N2, 30 min).* – changes are statistically significant (p<0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p<0.05) between the group exposed to acute hypoxia and groups treated by succinate or α-ketoglutarate at acute hypoxia.
Nitric oxide system under the succinate and α-ketoglutarate impact during acute hypoxia
It is known that the ratio of metabolic transformation pathways largely depends on the type of hypoxia and the ability of the system to biosynthesize nitric oxide (NO). NO can play a regulatory role as an electron acceptor in the mitochondrial respiratory chain in conditions of reduced oxygen content, thereby maintaining the functional capacity of the mitochondria. For this purpose, we analyzed the content of nitrite, nitrate, carbamide, and putrescine in liver tissue, blood plasma, and erythrocytes in conditions of acute hypoxia and administration of SC or KGL. The data from this series of studies are presented in Fig. 2-5.
The parenteral effects of SC exposure in normoxia conditions were related to an increase in the nitrite anion content primarily in the blood of rats, namely, a significant increase in erythrocytes and a threefold increase in blood plasma (p < 0.01). A significant decrease in carbamide content in plasma (p < 0.01) under the SC impact with a simultaneous significant increase in its content in erythrocytes (p < 0.001) was observed, which may have been a result of the increase in the level of polyamines in erythrocytes, as shown in Fig. 2-3.
Regarding the effects of the KGL administration on the rats in normoxia conditions, we analyzed the increase in the content of nitrite and nitrate anions in the blood (erythrocytes and plasma) and liver relative to the untreated control. It should be noted that the percentage of the increase was significant only in plasma and was significantly lower than the values in the untreated rats. Probably, the decrease in the nitrite anion content was related to its conversion to nitrate anion, since we observed a significant increase in the content of the latter in erythrocytes against the background of an increase in urea content, but not in the value of the total polyamines. Thus, the increase in the content of nitrite anion, which is a stable metabolite of nitric oxide, in the blood of rats under the influence of SC, but not KGL, confirms the important role of this oxidation substrate in optimization of the impact on energy supply processes.
Thus, in the conditions of maintaining the initial level of oxygen in the inhaled mixture, oxidative metabolism prevailed during the oxidation of SC, associated with an increase in the role of the metabolic pathway of nitrite anion metabolism with a simultaneous decrease in the contents of carbamides and polyamines. In contrast, in normoxia conditions, a high level of production of nitrite and nitrate anions was maintained under the КGL impact, with a simultaneous increase in the metabolic non-oxidative transformations of urea and ornithine. However, the changes in the production of the latter indicator, established by changes in the total polyamines, were insignificant.
Acute hypoxia is accompanied by a significant decrease in the production of nitric oxide, which was assessed by the content of its stable metabolite nitrite anion and nitrate anion, and the contents of urea and polyamines in hepatic tissue are also likely to decrease (Fig. 4 and 5). Thus, a significant decrease in the oxygen content in the inhaled air accompanied by severe signs of tissue hypoxia inhibited the activity of NO synthase systems, significantly reducing the production of nitric oxide in the liver. In this study, a decrease in the content of nitrite anion in erythrocytes and an increase in its content in blood plasma were observed, which may be an important regulatory component of maintaining homeostasis in such extreme conditions (Fig. 2 and 3).
The effects of the SC exposure under hypoxia were accompanied by a significant increase in the content of nitrite anion in the plasma and erythrocytes of rats relative to the values obtained under acute hypoxia. The functional role of polyamines increases at the impact of SC under acute hypoxia, as evidenced by the results of our studies showing the reduction of content of carbamides in the blood (plasma and erythrocytes) and in the liver under hypoxia, with a simultaneous increase in the content of polyamines. In particular, the content of polyamines as an indicator of transformations in the urea to ornithine cycle increased primarily under the influence of SC in the blood, but not in the liver. It can be concluded that exposure to exogenous SC + acute hypoxia significantly increased the production of nitric oxide in the liver relative to the values obtained under acute hypoxia. In these conditions, the content of carbamides also increased.
The impact of KGL under acute hypoxia was accompanied by a significant increase in the nitrite anion content, which was significantly higher than the values obtained when SC was administered. At the same time, there was no decrease in the contents of urea and polyamines relative to the values obtained under acute hypoxia. Thus, the KGL administration caused an increase in nitric oxide production, which can act as an important limiting factor in the intensification of reactive oxygen species (ROS) generation. These dependencies were shown earlier in the analysis of mitochondrial respiration inhibition.
The comparative analysis of nitric oxide production assessed through its stable metabolites (nitrites and nitrates) in relation to the effects of SC and KGL and hypoxia yielded the results described below. We calculated the ratio of the sum of carbamides and polyamines and the ratio of nitrite to the sum of nitrite and nitrate anions (Fig. 5). It should be noted that the formation of nitrite anion as a metabolite of NO was found to be higher under the influence of KGL compared to the effects of SC. In particular, under the influence of SC, the content of nitrite anion increased by 180.6% (p<0.001), whereas the increase obtained in the case of KGL was significantly higher, i.e. 269.7% (p<0.001). Under the impact of SC, a portion of nitric oxide from stimulated production in the liver may undergo irreversible transformations to carbamide, as demonstrated by the increase in the carbamide content evidenced by the increase in the sum of carbamide and polyamines (Fig. 5). On the contrary, under the influence of KGL, the role of NO produced in the liver increased, as demonstrated by the significantly increased ratio of nitrite to the sum of nitrite and nitrate anions in the liver. Thus, under the influence of exogenous KGL compared to SC in the liver, there was an increase in nitric oxide production in terms of its stable metabolite nitrite anion, indicating an increased role of oxidation of this metabolite of the Krebs cycle under hypoxia.
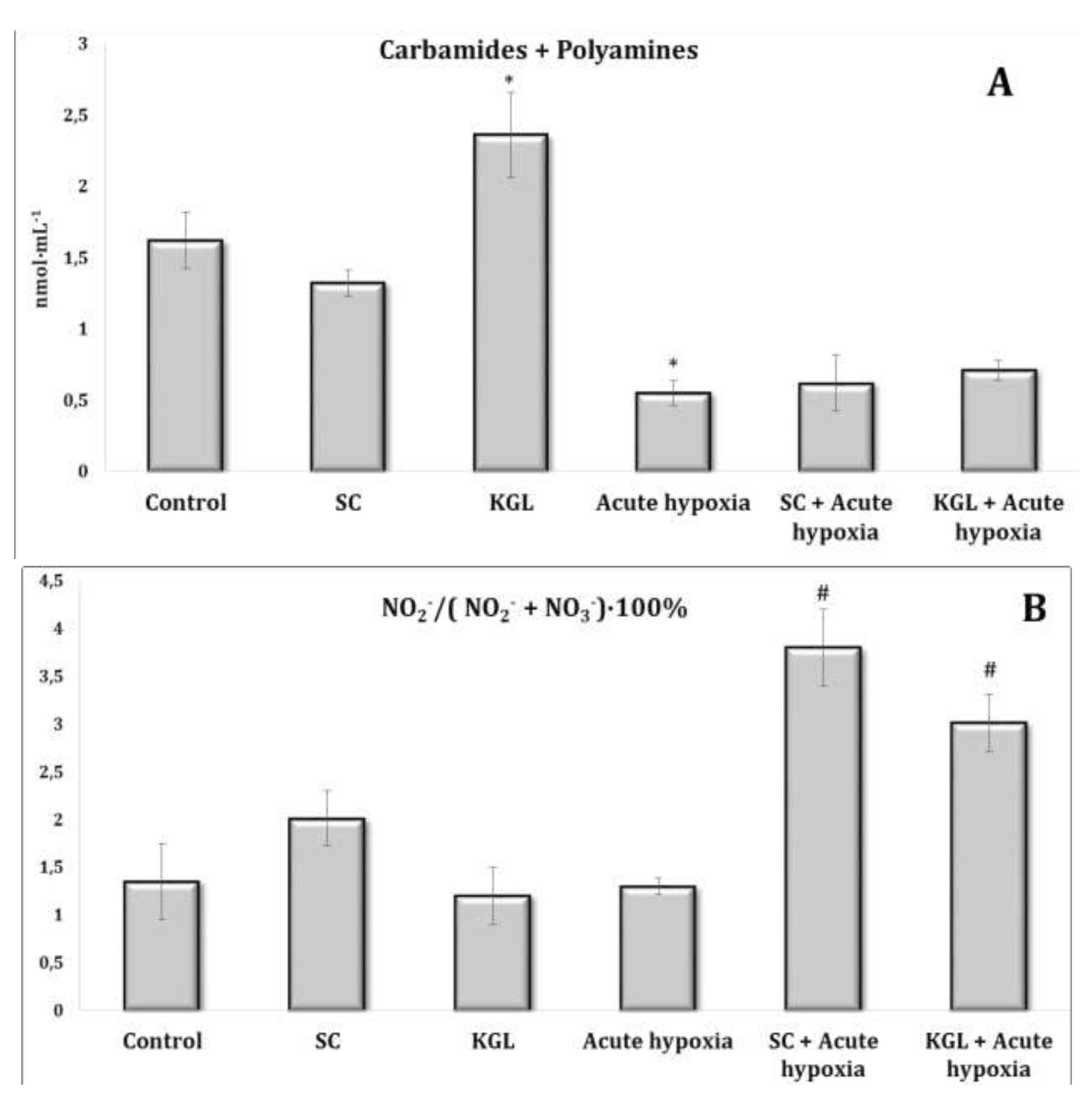
Fig. 2:Effects of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on carbamides and summary polyamines ratio (A), and nitrite to nitrite and nitrate ratio (B) [NO2–/(NO2– + NO3–)·100%] in the erythrocytes of rats (n = 6) at acute hypoxia (7% O2 in N2, 30 min).* – changes are statistically significant (p<0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p<0.05) between the group exposed to acute hypoxia and groups treated by succinate or α-ketoglutarate at acute hypoxia.
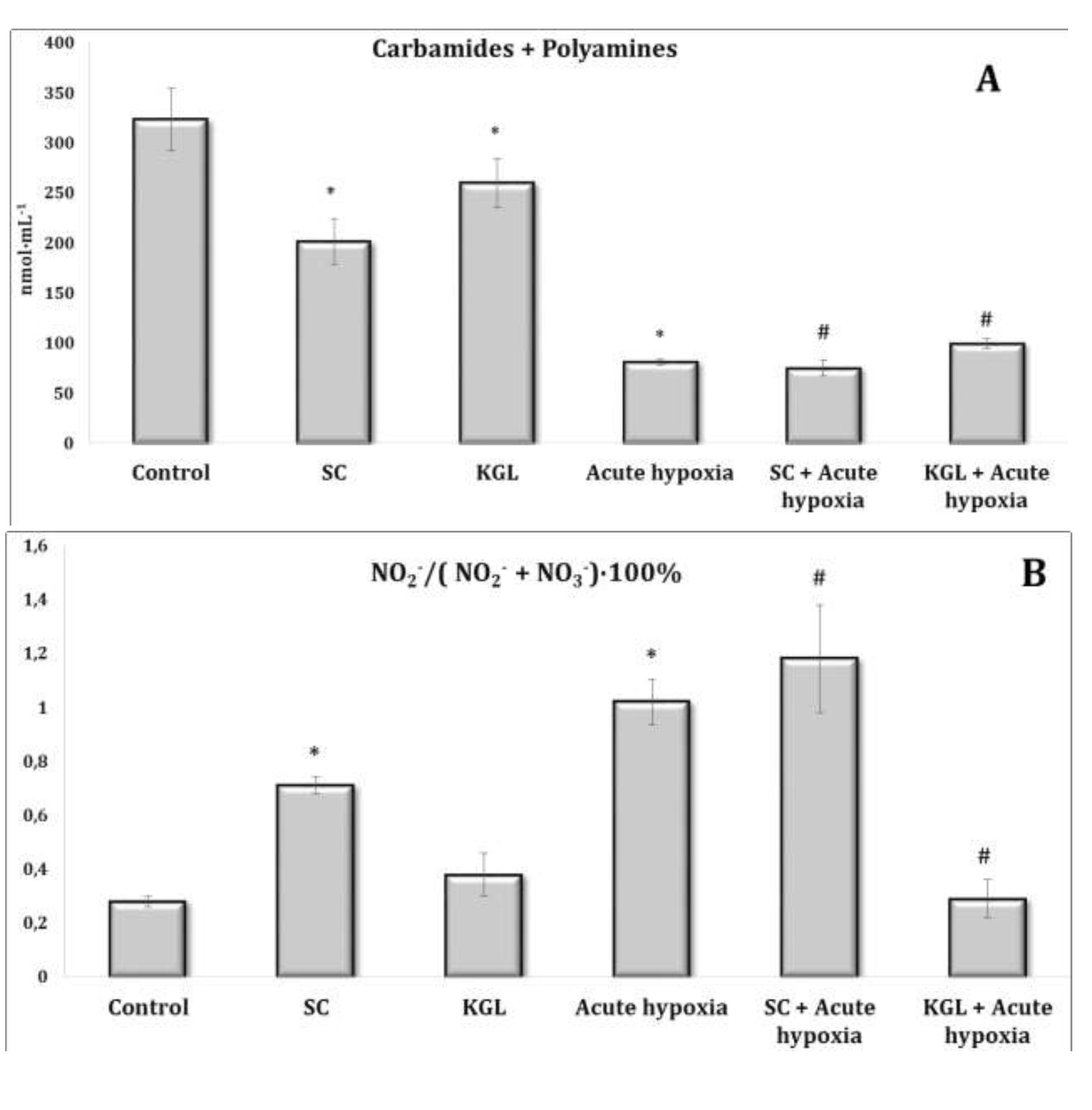
Fig. 3: Effects of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on carbamides and summary polyamines ratio (A) and nitrite to nitrite and nitrate ratio (B) [NO2–/(NO2– + NO3–)·100%] in the plasma of rats (n = 6) at acute hypoxia (7% O2 in N2, 30 min).* – changes are statistically significant (p<0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p<0.05) between the group exposed to acute hypoxia and groups treated by succinate or α-ketoglutarate at acute hypoxia.
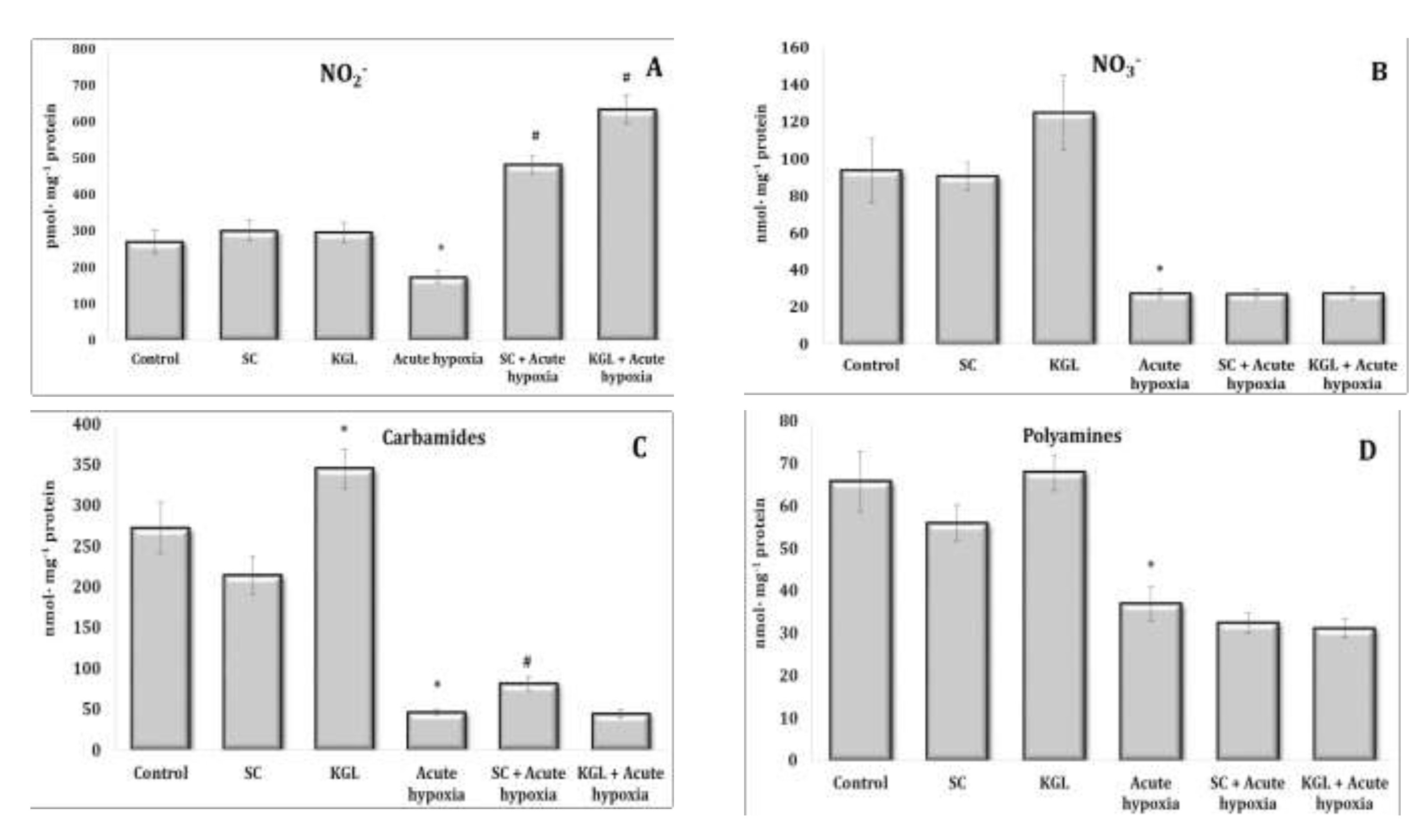
Fig. 4: Effects of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on levels of nitrites (A, pmol∙mg-1 protein), nitrates (B, nmol∙mg-1 protein), carbamides (C, nmol∙mg-1 protein) and summary polyamines (D, nmol∙mg-1 protein) in the liver of rats (n = 6) at acute hypoxia (7% O2 in N2, 30 min).* – changes are statistically significant (p<0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p<0.05) between the group exposed to acute hypoxia and groups treated by succinate or α-ketoglutarate at acute hypoxia.
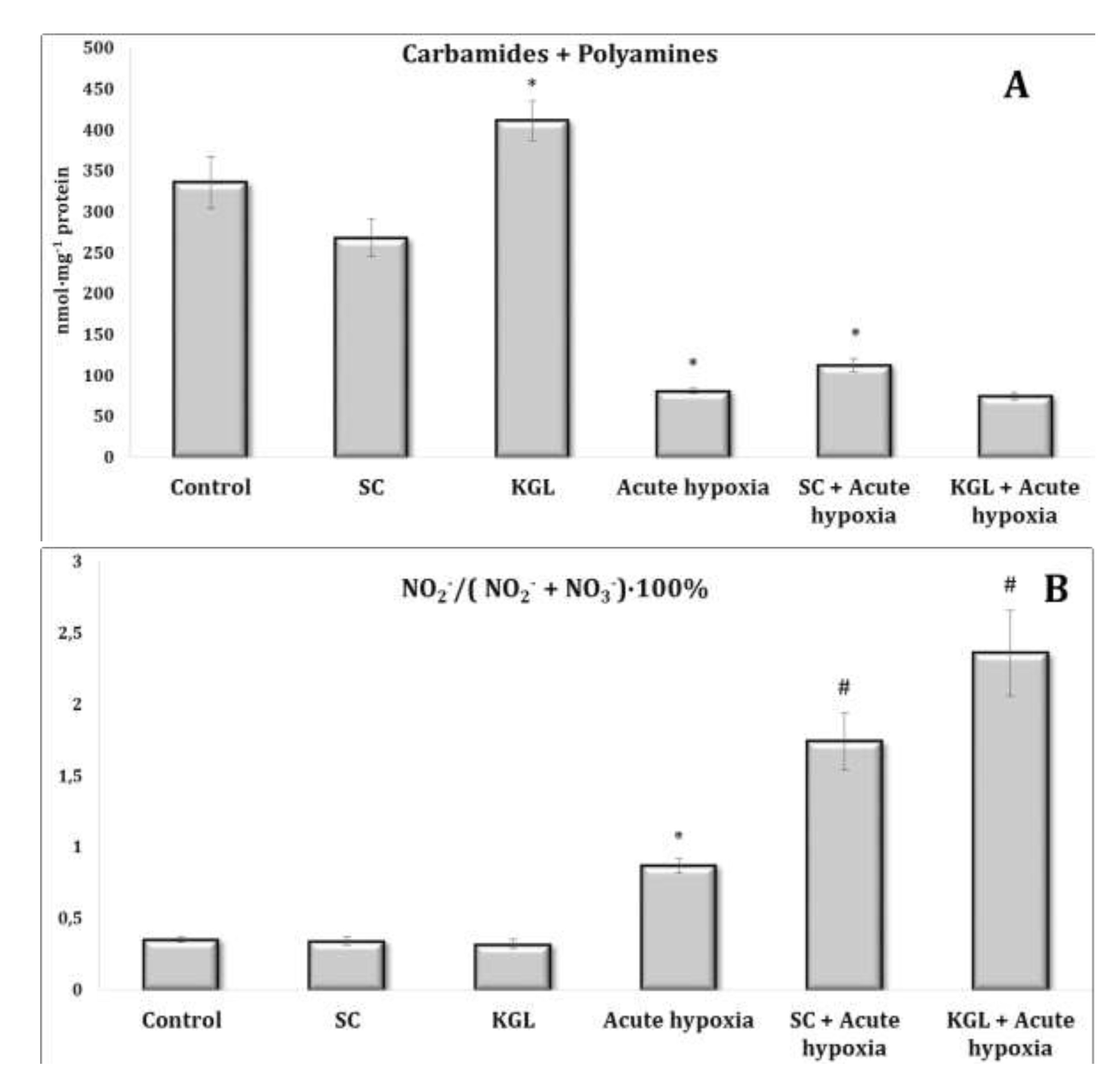
Fig. 5: Effects of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on carbamides and summary polyamines ratio (A), nitrite to nitrite, and nitrate ratio (B) [NO2–/(NO2– + NO3–)·100%] in the liver of rats (n = 6) at acute hypoxia (7% O2 in N2, 30 min).* – changes are statistically significant (p<0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p<0.05) between the group exposed to acute hypoxia and groups treated by succinate or α-ketoglutarate at acute hypoxia.
Biomarkers of lipid peroxidation, oxidative modification of proteins, and antioxidant defenses
It is known that the significant dependence of NOS activity on the presence of oxygen causes an increase in superoxide anion production, which, in turn, may cause an increase in the intensity of lipid peroxidation processes under hypoxia. Therefore, we studied the content of TBARS as the end products of lipid peroxidation and the levels of aldehydic and ketonic derivatives of oxidatively modified proteins when both Krebs cycle intermediates (SC and KGL) were administered, and when these drugs were administered before the acute hypoxia session. These data are summarized in Table 4.
In turn, under normoxia, we did not observe statistically significant differences between the studied parameters, and the data were within the statistical error of the experiment. The acute hypoxia significantly affected the processes of lipid peroxidation and protein modification, which was expressed in the statistically significant intensification of these processes, as assessed by the level of TBARS as well as aldehydic (AD OMP) and ketonic derivatives (KD OMP) of oxidatively modified proteins. The administration of both SC and KGL before acute hypoxia significantly decreased the levels of biomarkers of oxidative stress, but these values in the KGL variant were more significant for both blood plasma and hepatic tissue, compared with the data obtained under acute hypoxia. Thus, it is possible to highlight the more pronounced protective effects of KGL under acute hypoxia than the effects of SC. These changes can be associated with different effects of these drugs on the system of antioxidant defenses, namely the activity of superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx) presented in Table 5.
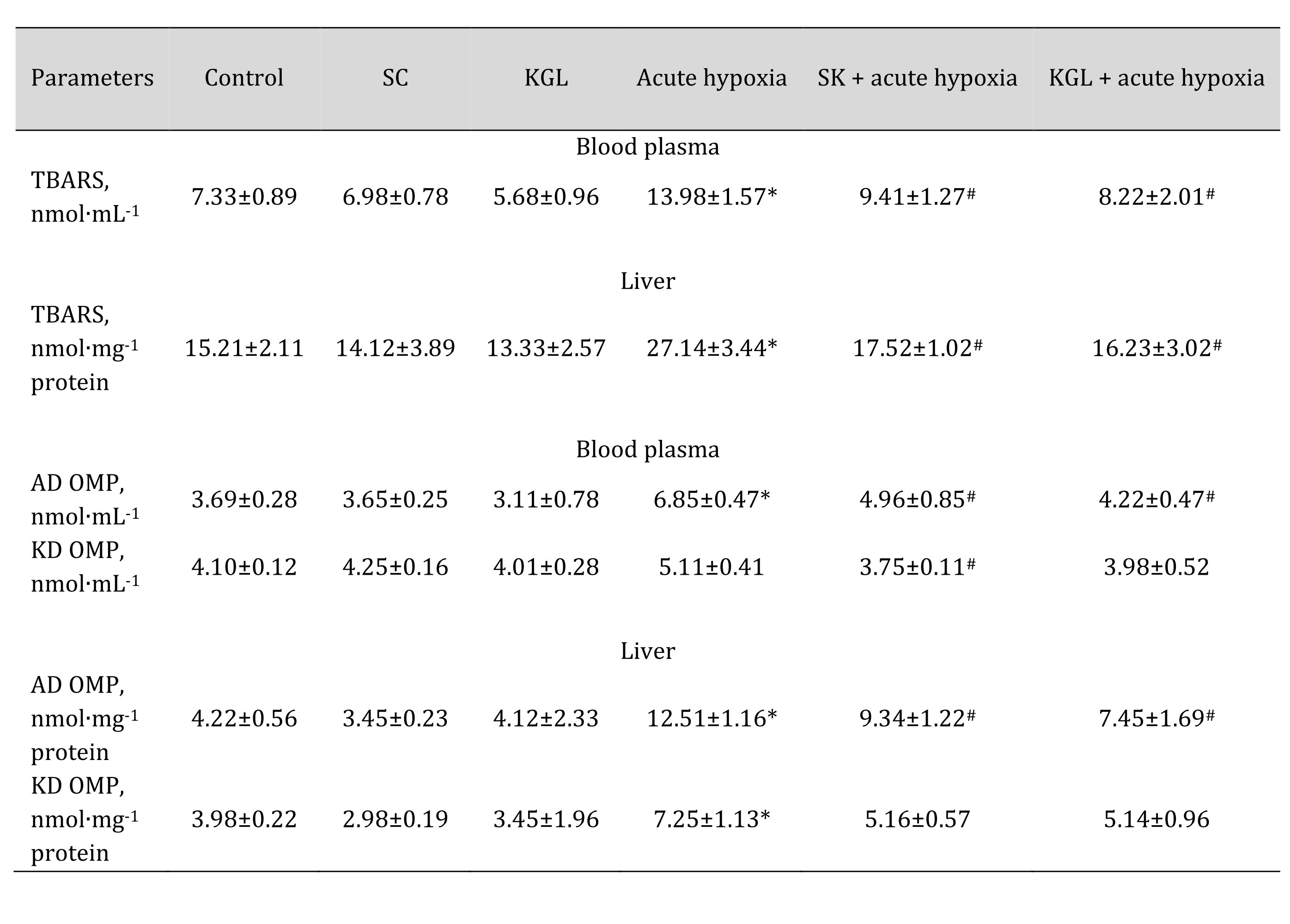
Table 4: Effect of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on levels of biomarkers of lipid peroxidation estimated as TBARS (nmol∙mL-1), aldehydic and ketonic derivatives of oxidatively modified proteins in the plasma and liver of rats at acute hypoxia. * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p < 0.05) between the group exposed to acute hypoxia and groups treated with succinate or α-ketoglutarate under acute hypoxia
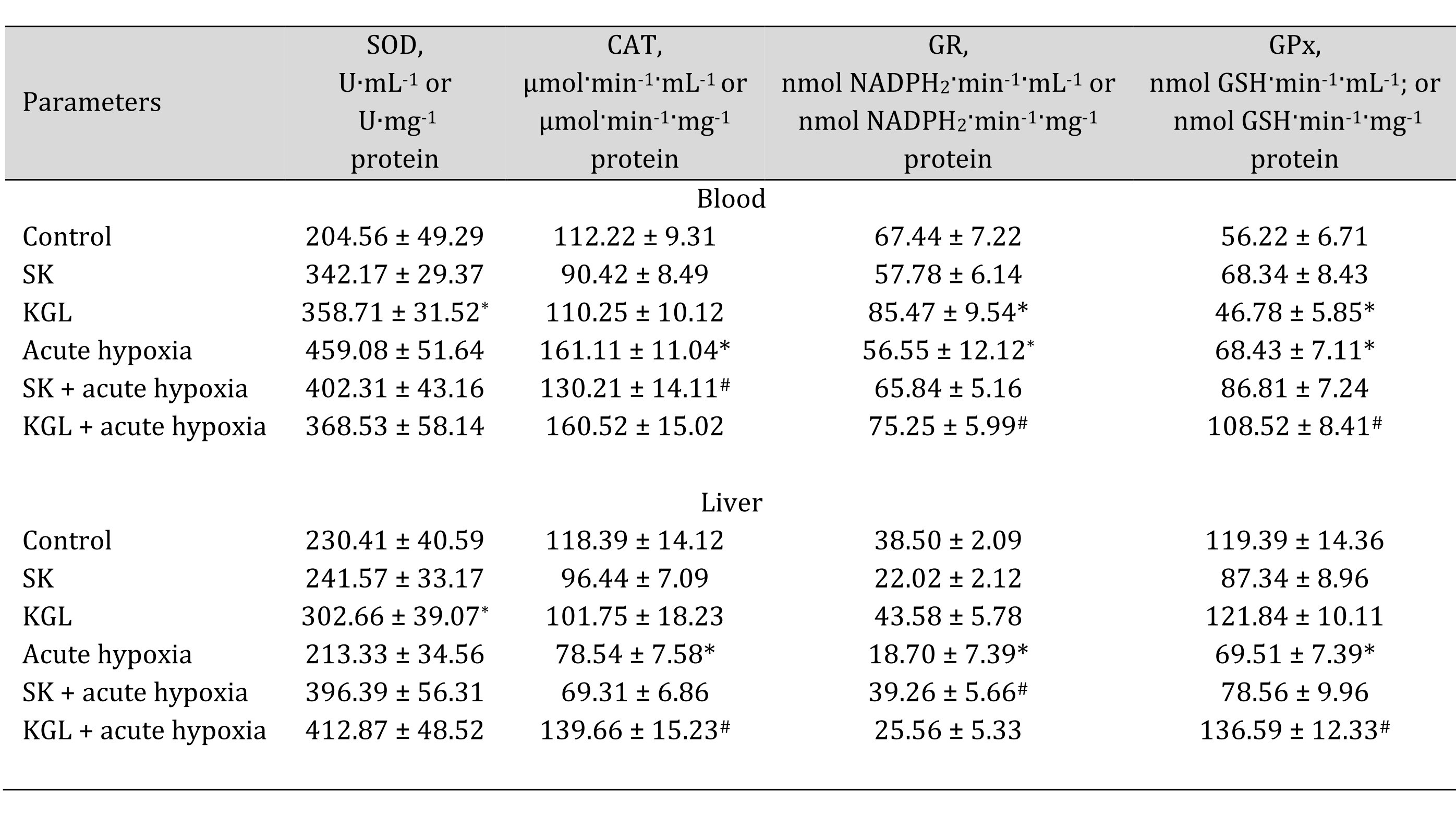
Table 5: Effects of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on activities of antioxidant enzymes in the blood and liver of rats at acute hypoxia. * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p < 0.05) between the group exposed to acute hypoxia and groups treated with succinate or α-ketoglutarate under acute hypoxia
Balance of acetylcholine and catecholamines
Changes in the content of acetylcholine (ACh) and cholinesterase (AChE) activity as well as the content of such catecholamines as adrenaline (AD), norepinephrine (NA), dopamine (DA), and dioxyphenylalanine (DOPA) in the whole blood of rats exposed to acute hypoxia and parenteral administration of KGL and SC are shown in Fig. 6. It was found that the exposure to extreme factors, acute hypoxia in particular, causes a state of functional stress in the rat organism, which is accompanied by an increase in the hormonal and mediator links of the sympathoadrenal system. Thus, the oxygen-dependent deficiency condition caused a sharp increase in the blood content of catecholamines and their precursors (Fig. 7) with a simultaneous increase in the role of parasympathetic nervous regulation.
A significant increase in the content of ACh is accompanied by a significant increase in the activity of the enzyme participating in its inactivation. The administration of KGL before the acute hypoxia session led to an increase in the regulatory link of parasympathetic activation, as it significantly increased the content of ACh in the blood with a decrease in the activity of the enzyme of its hydrolysis (Fig. 6). In particular, 30 minutes of exposure of the rats to acute hypoxia caused a significant increase in the blood concentration of AD and NA, compared to the group of the control untreated animals. The content of DA increased especially significantly in these conditions. The effects of the parenteral exposure to KGL under acute hypoxia were associated with a decrease in the content of all catecholamines except DOPA, compared with the group of animals exposed to acute hypoxia. The administration of SC in these conditions was accompanied by a significant decrease in the content of AD and DA only. Thus, the administration of both KGL and SC to the rats resulted in a decrease in the blood concentration of catecholamines (Fig. 7). This effect of both drugs can be considered an effective factor of protection in stressful conditions accompanied by hypoxia and activation of ROS generation, which can inevitably damage cellular and mitochondrial membranes, as shown earlier in this paper. It should be noted that the exposure to KGL under acute hypoxia caused marked changes in the content of NA and DA, while SC changed only the content of AD. Therefore, it can be argued that the effect of limiting the role of catecholamines in the effects of KGL in the development of hypoxia-induced stress is more significant than the effects of SC in similar conditions.
After this series of studies, we concluded that KGL and SC can be used to correct oxygen-dependent processes (oxidative phosphorylation, microsomal oxidation, and lipid peroxidation) by affecting the nitric oxide system under acute hypoxia. The obtained results of the analysis of the effect of KGL, primarily in the development of acute hypoxia, are associated with changes in the processes of oxidative phosphorylation, which is a powerful tool for energy supply and is evident primarily in the functional state of mitochondria. The effects of KGL are aimed at reducing energy consumption and production of ROS with an increased efficacy of oxygen consumption processes and a decrease in the level of microsomal oxidation. This can also be observed in the case of the reduction of the intensity of lipid peroxidation under acute hypoxia as an important factor in the protective effect of KGL compared to SC.
Intermittent hypoxia training and oxygen-dependent processes.
After elucidating the basic mechanisms of oxygen-dependent processes in acute hypoxia, we decided to compare these data in relation to another type of hypoxic stress, namely intermittent hypoxia training (IHT), and to elucidate the role of endogenous KGL and SC in these processes. A comparative analysis of the effects of KGL and SC after IHT on mitochondrial respiration is presented in Table 6.
The analysis of mitochondrial respiration processes showed that, after the course of IHT, the parameters of mitochondrial functioning changed, which was especially clearly manifested in the post-testing of this group of animals by acute hypoxia. This was demonstrated by a significant decrease in respiratory processes in the state of oxygen consumption stimulated by ADP, an increase in the coupling between oxygen consumption and oxidative phosphorylation, and especially the efficiency of oxygen consumption (ADP-to-oxygen ratio). It can be assumed that the two-week course of adaptation to hypoxia in the experimental animals caused changes in mitochondrial functioning by redistributing the use of substrates in the respiratory chain, highlighting the effective oxygen-dependent mechanisms.
This thesis was also confirmed by the results of the analysis of the marker of microsomal oxidative processes connected with aminopyrine-N-demethylase activity in the rats exposed to IHT. We obtained a marked decrease in aminopyrine-N-demethylase activity both in the rats exposed to IHT and in the IHT-exposed rats after the acute hypoxia test (Fig. 8). The formation of mechanisms of adaptation to oxygen deficiency is associated with an increase in the efficiency of the NADPH oxidase pathway, which was revealed in the inhibitor assay with malonate (2 mM, an inhibitor of SDH) and rotenone (10 μM, an inhibitor of mitochondrial complex I) after IHT (data not shown).
The more complete profile of the studied changes can be revealed after clarifying the changes in the ratio of oxidative metabolism pathways (the sum of nitrite and nitrate, the ratio of nitrite to the sum of nitrite and nitrate) and non-oxidative metabolism (the sum of urea and polyamines formed). These changes are presented in Table 7. It has been shown that the formation of adaptive responses to the effects of IHT and to the subsequent effects of acute hypoxia is mediated by the role of NO-dependent mechanisms, which we assessed by the transformations in the metabolism of the nitrite, nitrate, carbamide, and total polyamines. Previously in this article, it was shown that under the acute hypoxic exposure, there was a significant decrease in the total content of carbamides and polyamines in the blood and hepatic tissue, while the adaptive mechanisms obtained with the IHT method were accompanied by a marked increase in the production of nitric oxide analyzed through its stable metabolites in tissues, i.e. nitrites and nitrates. It is possible that such an increase in the nitrite and nitrate pool shown for hepatic tissue may be due to the fact that increased NO levels under acute hypoxia can be scavenged through the increasing capacity of the NO system during adaptation. This was confirmed by the results of our study of the level of oxidative stress biomarkers and the activities of antioxidant enzymes in response to the acute hypoxia test of the rats that underwent the IHT adaptation (Table 8).
Thus, the analysis of oxygen-dependent processes at the level of mitochondrial phosphorylation, microsomal oxidation, and lipid peroxidation revealed that the biochemical reactions to acute hypoxia and the adaptive responses to the effects of intermittent hypoxia are mediated by SC and KGL through NO-dependent metabolic mechanisms assessed by changes in the pool of nitrates, nitrites, carbamide, and total polyamines. The activation of mitochondrial SC oxidation caused by acute hypoxia decreased in the animals after the course of IHT adaptation. Intermittent hypoxia, preventing the intensification of lipid peroxidation in the liver under the exposure of acute hypoxia, increased the efficiency and coordination of mitochondrial function in the liver by enhancing the role of NO-dependent regulatory mechanisms. The NADPH-oxidase pathway and the associated oxidation of KGL are limited in the initial stages of hypoxia and largely determined the impairment of the energy-synthesizing function of mitochondria. The formation of adaptive mechanisms to oxygen deficiency is associated with an increase in the efficiency of the NADPH oxidase pathway, as shown by the KGL oxidation.
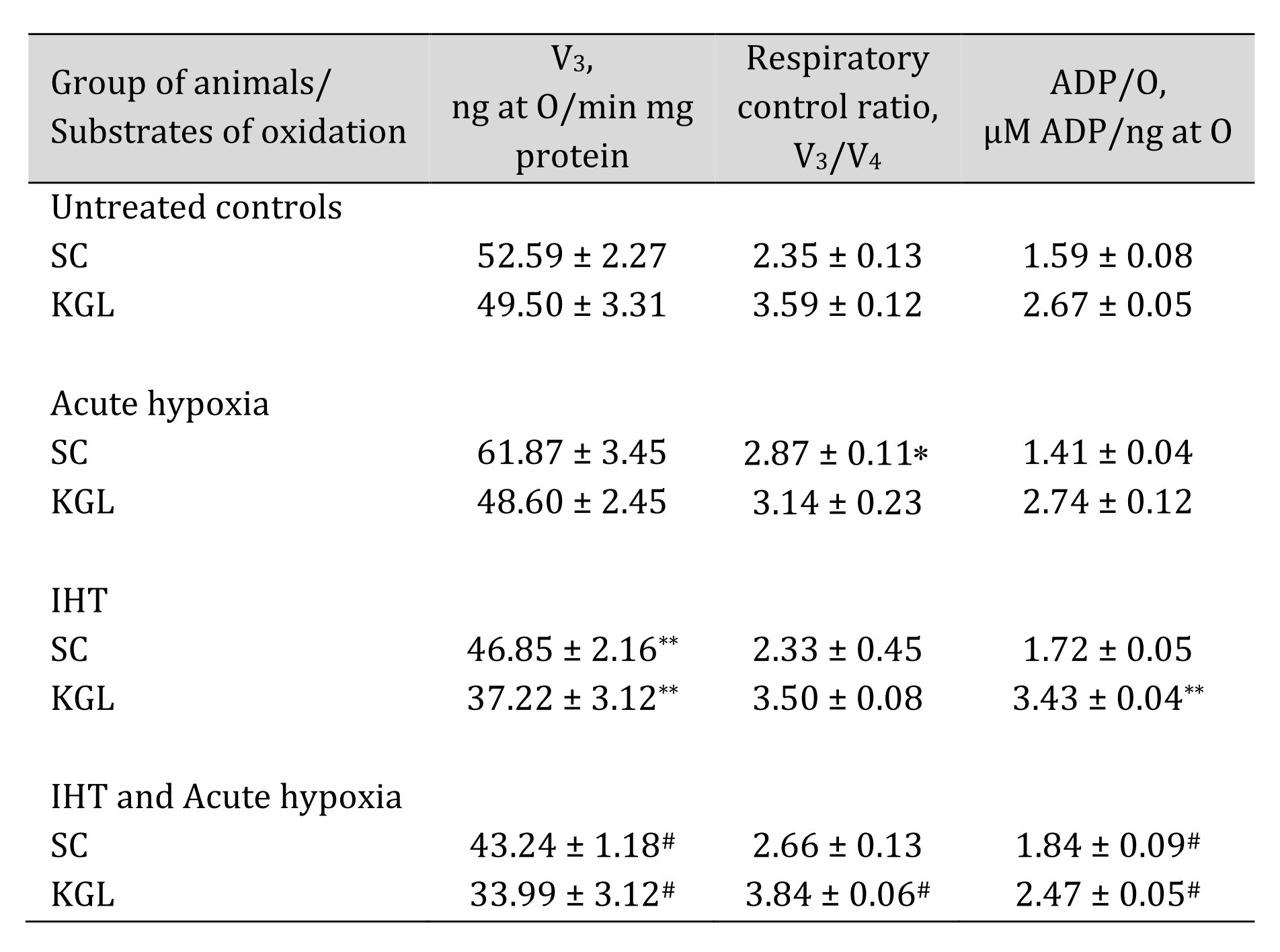
Table 6: Mitochondrial respiration in the liver at the presence of SC and KGL as substrates of oxidation during acute hypoxia (AH, 7% O2 in N2, 30 min) and after a course of 14 days of intermittent hypoxic training (IHT, 15-min 10% oxygen, 5 cycles per day) of rats (M ± m; n = 6). Oxygen uptake by hepatic mitochondria was measured polygraphically in the presence of 0.35 mM succinate or 1 mM α-ketoglutarate as substrates of mitochondrial respiration. As a phosphate acceptor, ADP was administrated at a concentration of 0.2 mM. * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; ** – changes are statistically significant (p < 0.05) between the untreated control group and the IHT group;# – changes are statistically significant (p < 0.05) between acute hypoxia or IHT-treated group and IHT and Acute hypoxia group
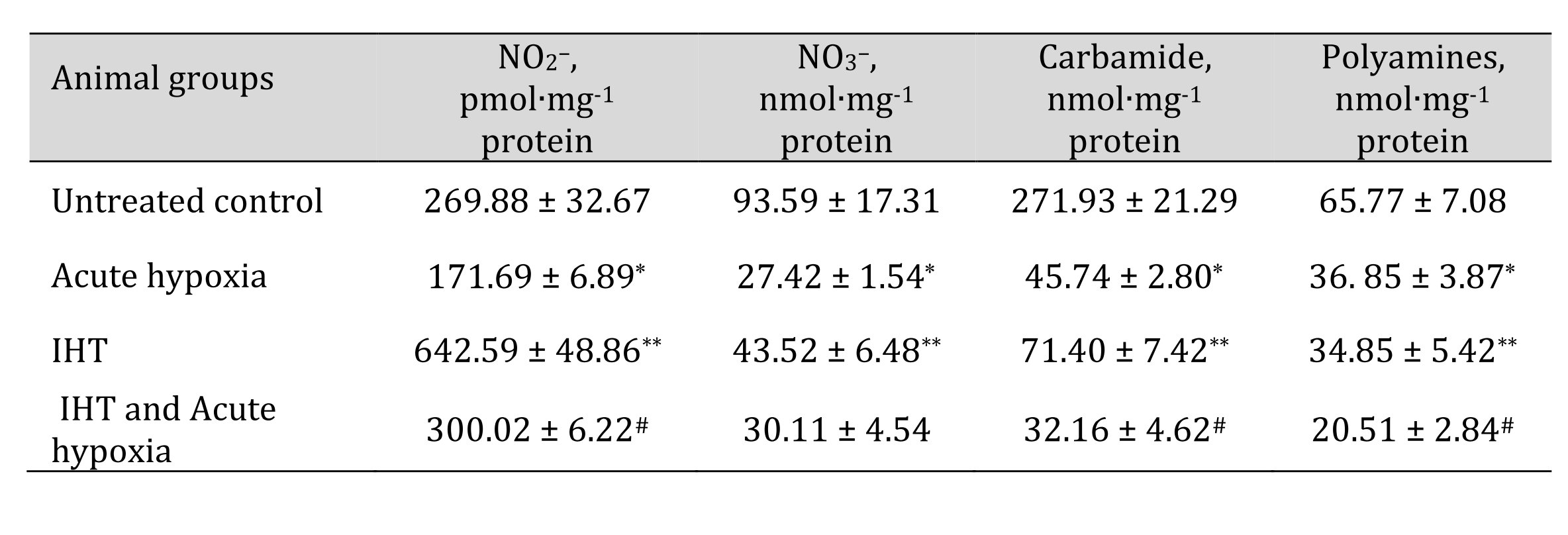
Table 7: The levels of nitrites, nitrates, urea, and polyamines in the liver of rats after acute hypoxia and intermittent hypoxia training (IHT) (M ± m, n = 6). * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; ** – changes are statistically significant (p < 0.05) between the untreated control group and the IHT group;# – changes are statistically significant (p < 0.05) between acute hypoxia or IHT-treated group and IHT and Acute hypoxia group
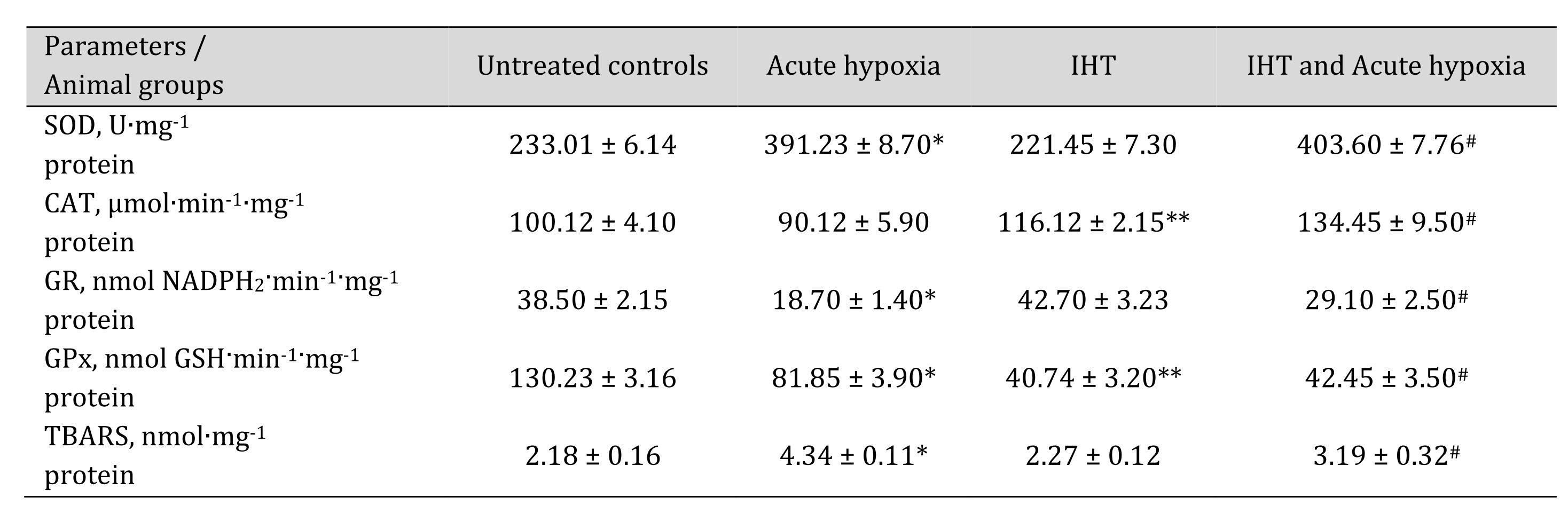
Table 8: Activities of antioxidant enzymes such as superoxide dismutase (SOD, U∙mg-1 protein), catalase (CAT, μmol∙min-1∙mg-1 protein), glutathione reductase (GR, nmol NADPH2·min-1·mg-1 protein), glutathione peroxidase (GPx, nmol GSH·min-1·mg-1 protein) and intensity of lipid peroxidation (TBARS, nmol∙mg-1 protein) in the liver of rats after acute hypoxia and intermittent hypoxia training (IHT) (M ± m, n = 6). * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; ** – changes are statistically significant (p < 0.05) between the untreated control group and the IHT group;# – changes are statistically significant (p < 0.05) between acute hypoxia or IHT-treated group and IHT and acute hypoxia group
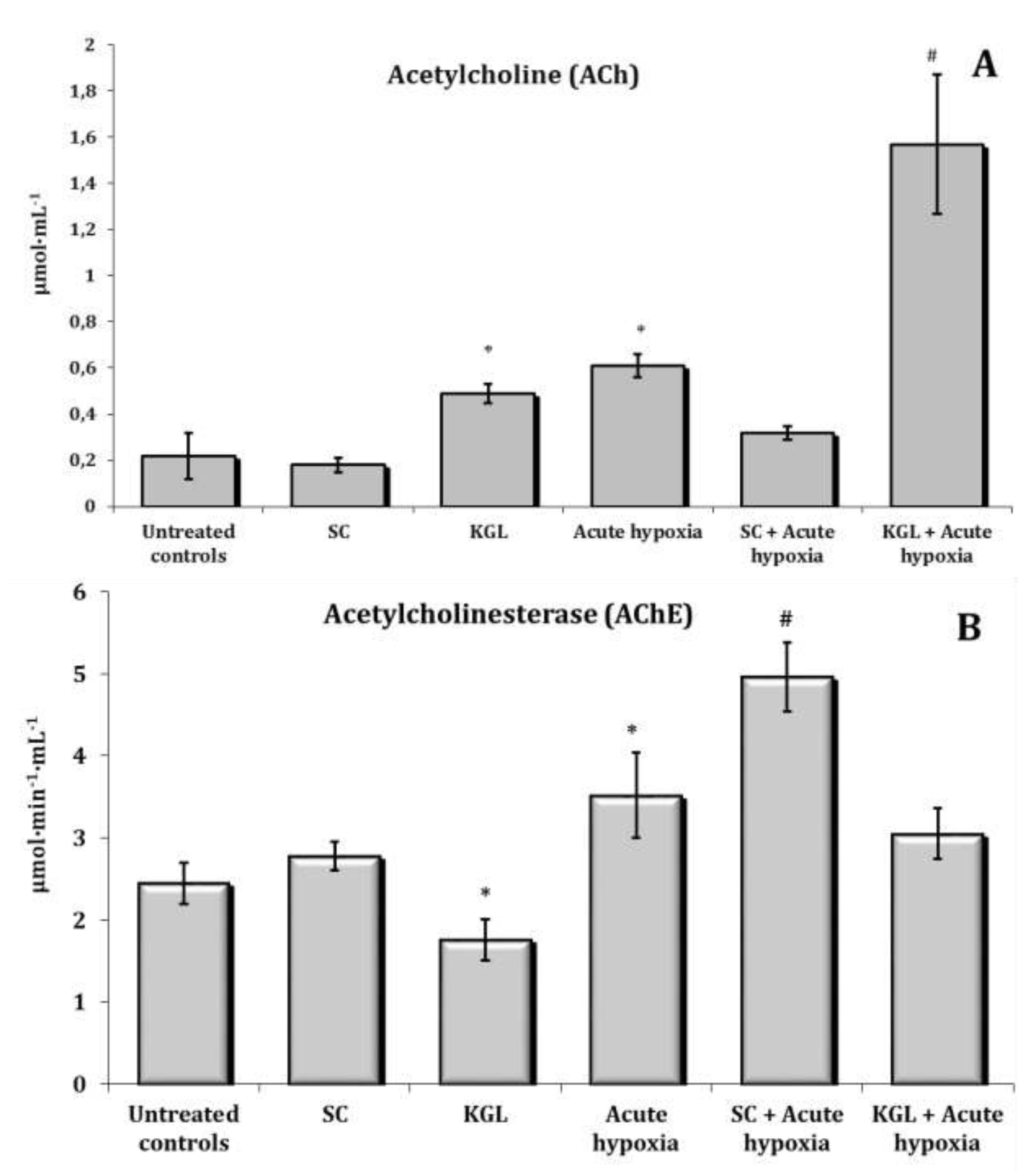
Fig. 6: Effects of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on acetylcholine level (A, μmol∙mL-1) and acetylcholinesterase activity (AChE, B, μmol∙min-1∙mL-1) in the blood of rats (n = 6) at acute hypoxia (7% O2 in N2, 30 min).* – changes are statistically significant (p<0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p<0.05) between the group exposed to acute hypoxia and groups treated by succinate or α-ketoglutarate at acute hypoxia.
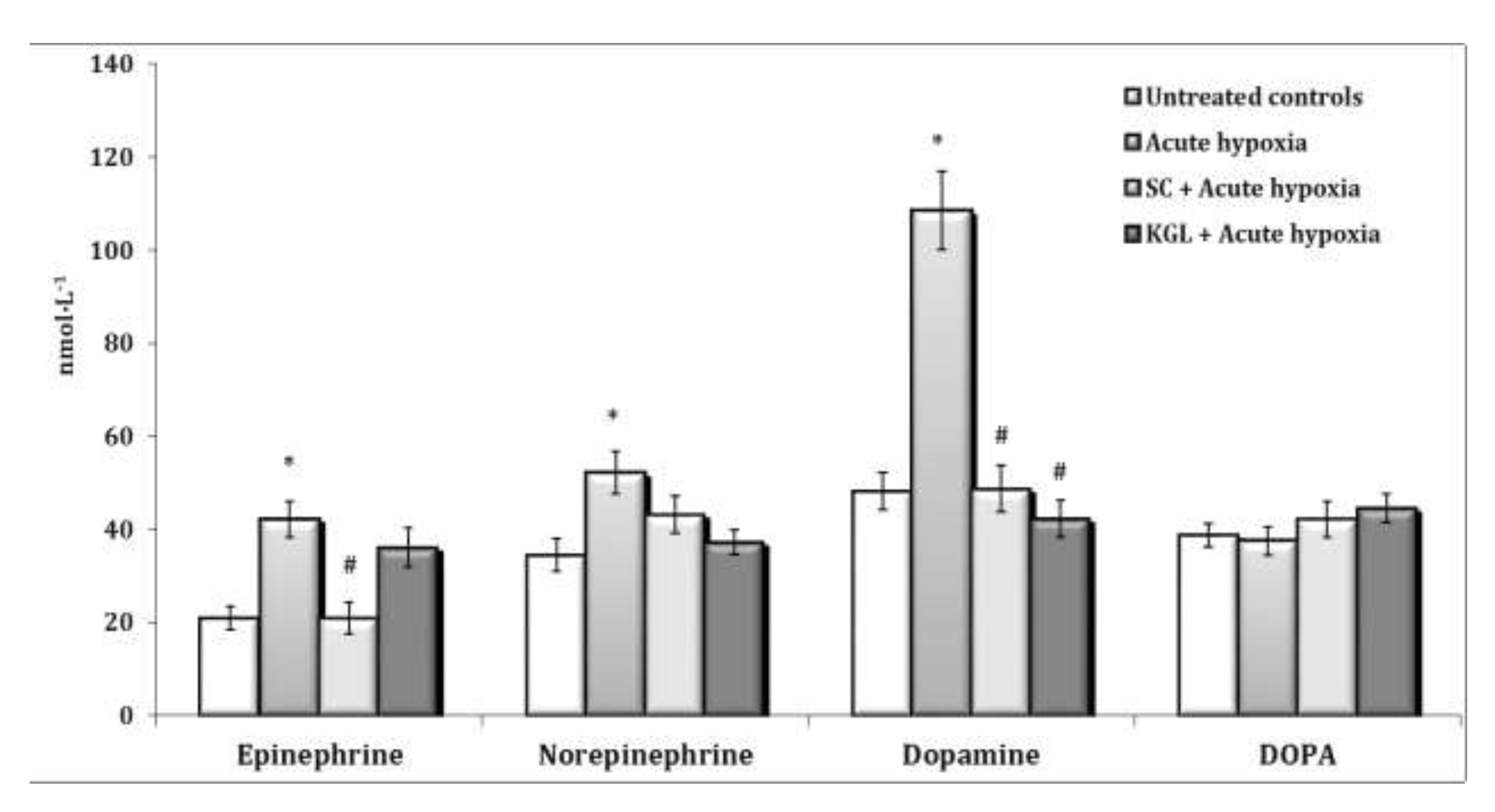
Fig. 7: Effects of succinate (SC, 50 mg/kg) and α-ketoglutarate (KGL, 200 mg/kg) on content of epinephrine, norepinephrine, dopamine and DOPA (nmol∙L-1) in the blood of rats (n = 6) at acute hypoxia (7% O2 in N2, 30 min).* – changes are statistically significant (p<0.05) between the untreated control group and the acute hypoxia group; # – changes are statistically significant (p<0.05) between the group exposed to acute hypoxia and groups treated by succinate or α-ketoglutarate at acute hypoxia.
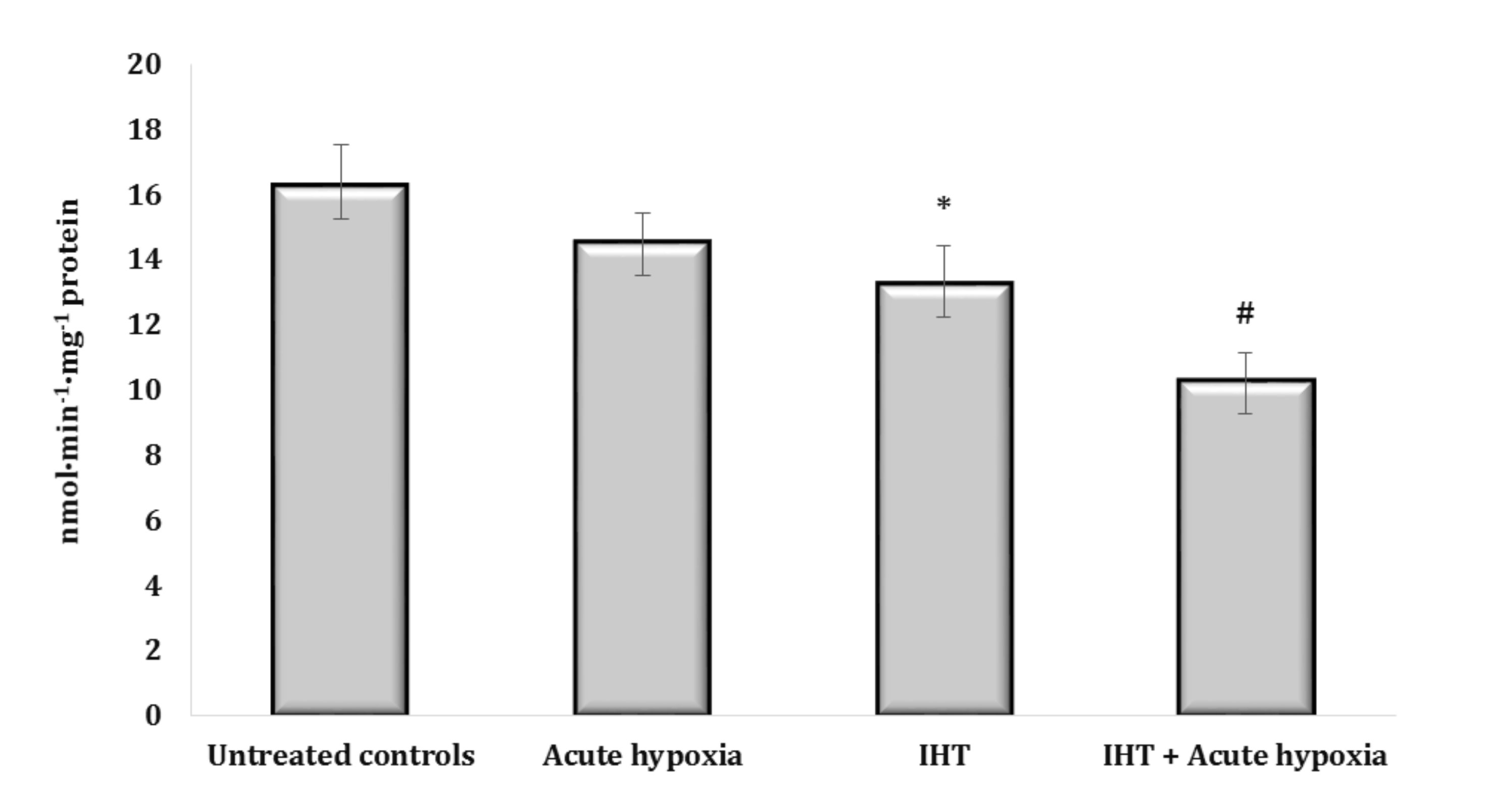
Fig. 8: Aminopyrine-N-demethylase activity (nmol∙min-1∙mg-1 protein) in the liver of rats (n = 6) after acute hypoxia (7% O2 in N2, 30 min) and intermittent hypoxia training (IHT) (M ± m, n = 6).* – changes are statistically significant (p<0.05) between the untreated control group and the IHT group; # – changes are statistically significant (p<0.05) between the group exposed to acute hypoxia and groups treated by IHT method at acute hypoxia.
Discussion
This study was focused on elucidation of the role of NO-dependent mechanisms of the anti-hypoxic protective role of the most important intermediates of the Krebs cycle (SC and KGL) in the blood and hepatic tissue of rats. Our work involves comparing our results with previous studies by other authors allowing elucidation of potential mechanisms and future research directions. We obtained important results that we would like to highlight in our conclusions.
Firstly, the use of KGL to correct the processes of energy supply by affecting the nitric oxide system at acute hypoxia caused changes in the functional state of mitochondria aimed at reducing energy consumption and ROS production while increasing the efficacy of oxygen consumption. Reduction of the intensity of lipid peroxidation and protein oxidation under acute hypoxia is an important factor in the protective effect of KGL, compared to SC. It is known that the significant dependence of NOS activity on the presence of oxygen leads to an increase in superoxide anion production, which, in turn, can lead to an increase in the intensity of lipid peroxidation under acute hypoxia [72, 73]. A decrease in the content of nitrite anion in erythrocytes and an increase in its content in blood plasma is an important regulatory component of homeostasis maintenance. It has been shown that NO can compete for superoxide anion with SOD to form peroxynitrite [74] and effectively reduce the level of oxidative stress.
Secondly, the relationship between the processes of energy supply, the intensity of oxidative stress, and the NO system under the influence of KGL was established; it is associated with a decrease in the negative manifestations of hypoxic damage caused by an increase in ROS and catecholamines. The important role of cholinergic structures in maintaining free radical homeostasis under acute hypoxia and the KGL administration was associated with the reciprocal enhancement of SC effects. SC reduced the negative manifestation of the effects of catecholamines and the associated activation of free radical oxidation under acute hypoxia, as has been reported by authors [75]. It was shown that SC increased mitochondrial oxidation via succinate dehydrogenase (SDH) activity and an elevation of mitochondrial membrane potential combined to drive mitochondrial ROS production. Thus, an increase in the efficiency of the NADPH-oxidase pathway is of fundamental importance in the formation of mechanisms of adaptation to oxygen deficiency [31], which we showed in the inhibition analysis of mitochondrial oxidative processes. Although this oxidation pathway is limited at the initial stages of hypoxia and largely determines the impairment of the energy-synthesizing function of mitochondria, our studies have shown that the increasing role of NOS reactions changed according to the catalytic properties and characteristics of mitochondrial complex I established earlier [30, 32, 8], which in turn contributes to the formation of long-term adaptive responses.
Thirdly, in contrast to SC action, the oxidation of KGL and other NAD-dependent substrates was less intense, while had a higher efficiency due to the activity of all coupling sites. In addition, the activation of oxidation of this substrate led to an increase in substrate phosphorylation and GTP synthesis. This condition is adequate to ensure anabolic and reducing processes that may be associated with the enhancement of cholinergic mechanisms of endogenous NO and the role of KGL in this effect. In the case of KGL, an increase in the ADP/O ratio and a pronounced efficacy of oxygen uptake under various extreme loads were investigated. Thus, the analysis of oxidative phosphorylation using SC and KGL as substrates for oxidation indicated the regulatory role of NO-dependent mechanisms [37, 36] at the level of oxidation of substrates of the mitochondrial respiratory chain, which can be used as a mechanism for regulating the activity of α-ketoglutarate and succinate dehydrogenases, i.e. important enzymes of the Krebs cycle. Previously, author [76] showed that mitochondrial complex I DNA mutations were the main genetic feature of oncocytic tumors, leading to the breakdown of respiratory complex I in vivo . These researchers associated such changes with the inability of oncocytic tumors to stabilize HIF1α and cause pseudohypoxia. The authors attribute this shift to a profound metabolic disorder and imbalance of α-ketoglutarate and succinate, which are responsible for stabilizing HIF1α.
In our study, the activation of KGL oxidation caused by the IHT method under the influence of a NO inhibitor can be considered an effective way to prevent the reduction of the role of NADH-dependent oxidation and enhancement of nitrite reductase mechanisms in acute hypoxia, without limiting the processes of free radical oxidation. It is possible that the increase in the concentration of nitric oxide during IHT limited the supply of reducing equivalents through mitochondrial complex I, determining the sensitivity of this complex to the inhibitory effect of NO on respiratory capacity, compared with the effect on mitochondrial complex II. The effectiveness of the IHT method has been shown by many authors as well [27, 77, 78]. The significant therapeutic potential of intermittent hypoxia also was reported in some studies [79, 80].
It is known that increased synthesis of hemoglobin and myoglobin induced by exogenous SC under hypoxia and an increase in the concentration of respiratory hemoproteins in blood and muscle under acute hypoxia contribute to an increase in both the capacity of the system for “fighting” for oxygen and the deposition of nitric oxide [36, 37]. Hemoglobin is of particular importance in this system. Its complexes with nitric oxide, formed as a result of the functioning of NO synthase and nitrite reductase systems [81] due to the inhibition of Ca2+-dependent NO synthase in the endothelium, and can have a vasodilator effect due to release from HbNO complexes [82]. Under hypoxia, the functional role of polyamines (spermine, spermidine, putrescine) as regulators of citrulline and arginine-succinate synthesis increase under the influence of SC, as shown in our study. The recycling of citrulline to L-arginine helps maintain the functional capacity of the urea cycle in the liver, which is associated with the functioning of the NO cycle [83]. This is evidenced by the results of our studies on the reduction of the carbamide content in the blood under acute hypoxia and in the liver against the background of an increase in the polyamine content. In particular, the content of polyamines as an indicator of transformations in the urea to ornithine cycle increases primarily under the influence of SC in the blood, but not in the liver, as revealed in our investigations.
Fourthly, the studied increase in the efficiency of mitochondrial functioning in the liver was primarily related to the possibility of switching to oxygen-independent metabolic pathways. This was associated with the L-arginase pathway of metabolic transformations [84]. The reduction of the role of oxygen-dependent NOS mechanisms under hypoxia modified by the dominant oxidation of SC and increasing the role of SDH allowed switching to L-arginase metabolic pathways associated with an increase in the role of KGL. In these conditions, the cholinergic mechanism of regulation and cGMP-dependent reactions of oxygen consumption were activated.
Fifthly, the use of KGL to correct the processes of energy supply by affecting the NO system at acute hypoxia caused changes in the functional state of mitochondria, aimed at reducing energy consumption and ROS production, while increasing the efficacy of oxygen consumption. Reduction of the intensity of oxidative stress under hypoxia is an important factor in the protective effect of KGL compared to SC. The relationship between the processes of energy supply, the intensity of oxidative stress, and the nitric oxide system was established under the influence of KGL, which was associated with a decrease in the negative manifestations of hypoxic damage caused by an increase in the content of catecholamines. The correlative analysis demonstrated a correlation between the increase in the content of nitrite anion and carbamides (r = 0.72, p < 0.05) under the influence of SC as well as nitrite anion, carbamides, and polyamines under the influence of KGL in blood erythrocytes (r = 0.72-0.93, p < 0.05), where high correlative relationships were observed as well. However, the values of the correlation coefficient were higher for the effects of the KGL administration compared to the effects of the SC administration. In the blood plasma, such a relationship between the content of nitrite anion and carbamides was shown for the effects of SC (r = 0.59, p < 0.05) and KGL (r = 0.84, p < 0.05). In the liver, positive correlations between all the studied parameters (r = 0.68-0.78, p < 0.05) were found for the effects of KGL. For the effects of KGL, there were correlations between the content of nitrite anion and carbamides (r = 0.74, p < 0.05) as well as polyamines (r = 0.96, p < 0.05).
Acute hypoxia was shown to cause a significant decrease in the activity of microsomal oxidation in the liver, which accounted for 88.8% (p < 0.05) of the control values. The parenteral administration of KGL before the acute hypoxia session did not change the activity of microsomal oxidation processes, which were significantly activated by SC. In particular, under the influence of SC, the aminopyrine-N-demethylase activity statistically increased. Thus, in the conditions of acute hypoxia, the increase in the activity of the microsomal oxidation system under the influence of exogenous SC may contribute to enhancement of the biotransformation of harmful compounds. The possibility of additional electron transfer from the NADH-cytochrome b5 reductase system of the outer mitochondrial membrane to the cytochrome oxidase chain of the inner mitochondrial membrane is also possible. This pathway makes it possible to regulate the [NADH]/[NAD+] ratio in the cytoplasm. A system of electron transfer from the NADH-specific chain of microsomes to the mitochondrial cytochrome oxidase system at the level of cytochromes c and b5 was established earlier [85, 86]. Thus, acute oxygen deficiency activated the hypothalamus-pituitary-adrenal system, causing marked changes in the ratio of catecholamines and a multistage shift in humoral and hormonal regulatory systems [87]. Due to the generalized excitation of these systems, these amines are involved in specific and nonspecific reactions of the organism to extreme environmental factors [88]. It has been shown that adaptation to periodic hypoxia in organs and tissues changes the expression of genes encoding different forms of hypoxia [89-91], expression of genes encoding different forms of NO synthase, and vascular NO-dependent reactions [92, 93]. Adaptation to hypoxia may be associated with activation of endogenous NO-dependent antiapoptotic mechanisms [94]. The fundamental ability of NO to suppress protein apoptosis has been demonstrated on several cellular targets, including endothelial cells, lymphocytes, and eosinophils [95, 96].
The results obtained indicate a correlation between the content of mediator substances, the antioxidant defenses, and the use of various Krebs cycle substrates. This is consistent with the activation of KGL oxidation as a precursor of SC and the increased adaptive capabilities of rats in acute hypoxic conditions. The latter indicates the important role of Krebs cycle substrates (SC and KGL, respectively), in the formation of short-term and long-term adaptive reactions of the organism to the effects of extreme loads accompanied by hypoxia. Our work revealed future directions of research related to the practical use of the results for targeted supplementation with these compounds to correct the adaptive capabilities of organisms in conditions accompanied by hypoxia of different origins.
Conclusion
Summarizing, the Krebs cycle intermediates such as SC and KGL significantly affect the functional changes in the nitrite and nitrate components of the nitric oxide system through multidirectional effects in the blood and liver. The SC effect in the blood, in particular in erythrocytes, on the production of nitrite anion as a stable nitric oxide metabolite and changes in the urea cycle functioning and the polyamine content was found to be higher than that of KGL. The administration of KGL to correct the processes of energy supply by affecting the nitric oxide system under acute hypoxia caused changes in the functional state of mitochondria, which are aimed at reducing energy consumption and production of ROS while increasing the efficacy of oxygen consumption. The multifactorial influence of the selected intermediates of the Krebs cycle on changes in the content of nitrite and nitrate in the blood and liver and the associated urea and ornithine cycle, which we identified, can modify the state of the energy supply system and may be one of the mechanisms through which nitric oxide exerts its effect on cellular mechanisms under the influence of extreme loads.
Acknowledgements
The current study was financially supported by the Pomeranian University in Słupsk and T.G. Shevchenko National University “Chernihiv Collegium”, Chernihiv, Ukraine.
Authors’ contributions
The authors contributed to the following aspects of the investigation: Conceptualization: NK, HT, OL; Data curation: NK, HT; Formal analysis: NK, HT; Investigation: NK, HT, OL; Methodology: NK, HT; Supervision: NK, HT; Writing – original draft: NK, HT; Writing – review and editing: NK, HT.
Statement of Ethics
The experiments were conducted in compliance with the Guidelines of the European Union Council, the current laws in Poland and Ukraine, and the recommendations of the Ethical Commission. Animal studies were conducted in accordance with the guidelines for Animal Research: Reporting of In vivo Experiments (ARRIVE) developed by the National Center for Replacement, Refinement and Reduction of Animals in Research (NC3R). They were approved by the Ethical Commission of T.G. Shevchenko National University “Chernihiv Collegium” (05/02/2019). The experiment was carried out in accordance with both the Directive 2010/63/EU on the protection of animals used for scientific purposes and with the Polish Act of 15 January 2015 on the protection of animals used for scientific or educational purposes (JL, February 26, 2015, Pos.266).
Funding
The present study was financially supported by the Pomeranian University in Słupsk and T.G. Shevchenko National University “Chernihiv Collegium”, Chernihiv, Ukraine.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
| 1 | Hand SC, Menze MA: Mitochondria in energy-limited states: mechanisms that blunt the signaling of cell death. J Exp Biol 2008;211:1829-1840.
https://doi.org/10.1242/jeb.000299 |
| 2 | Chandel NS: Evolution of Mitochondria as Signaling Organelles. Cell Metab 2015;22:204-206.
https://doi.org/10.1016/j.cmet.2015.05.013 |
| 3 | Song Y, Li C, Liu G, Liu R, Chen Y, Li W, Cao Z, Zhao B, Lu C, Liu Y: Drug-Metabolizing Cytochrome P450 Enzymes Have Multifarious Influences on Treatment Outcomes. Clin Pharmacokinet 2021;60:585-601.
https://doi.org/10.1007/s40262-021-01001-5 |
| 4 | Martínez-Reyes I, Diebold LP, Kong H, Schieber M, Huang H, Hensley CT, Mehta MM, Wang T, Santos JH, Woychik R, Dufour E, Spelbrink JN, Weinberg SE, Zhao Y, DeBerardinis RJ, Chandel NS: TCA Cycle and Mitochondrial Membrane Potential Are Necessary for Diverse Biological Functions. Mol Cell 2016;61:199-209.
https://doi.org/10.1016/j.molcel.2015.12.002 |
| 5 | Schultz BE, Chan SI: Structures and proton-pumping strategies of mitochondrial respiratory enzymes. Annu Rev Biophys Biomol Struct 2001;30:23-65.
https://doi.org/10.1146/annurev.biophys.30.1.23 |
| 6 | Sousa JS, D'Imprima E, Vonck J: Mitochondrial Respiratory Chain Complexes. Subcell Biochem 2018;87:167-227.
https://doi.org/10.1007/978-981-10-7757-9_7 |
| 7 | Martínez-Reyes I, Chandel NS: Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 2020;11:102.
https://doi.org/10.1038/s41467-019-13668-3 |
| 8 | Lukyanova LD, Kirova YI, Germanova EL: The Role of Succinate in Regulation of Immediate HIF-1α Expression in Hypoxia. Bull Exp Biol Med 2018;164:298-303.
https://doi.org/10.1007/s10517-018-3976-2 |
| 9 | Sharafati-Chaleshtori R, Shirzad H, Rafieian-Kopaei M, Soltani A: Melatonin and human mitochondrial diseases. J Res Med Sci 2017;22:2.
https://doi.org/10.4103/1735-1995.199092 |
| 10 | Semenza GL: Hypoxia-inducible factors in physiology and medicine. Cell 2012;148:399-408 https://doi.org/10.1016/j.cell.2012.01.021.
https://doi.org/10.1016/j.cell.2012.01.021 |
| 11 | Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB: ATP-citrate lyase links cellular metabolism to histone acetylation. Science 2009;324:1076-1080.
https://doi.org/10.1126/science.1164097 |
| 12 | Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT: Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 2000;275:25130-25138.
https://doi.org/10.1074/jbc.M001914200 |
| 13 | Kaelin WG Jr, Ratcliffe PJ: Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008;30:393-402.
https://doi.org/10.1016/j.molcel.2008.04.009 |
| 14 | Tennant DA, Frezza C, MacKenzie ED, Nguyen QD, Zheng L, Selak MA, Roberts DL, Dive C, Watson DG, Aboagye EO, Gottlieb E: Reactivating HIF prolyl hydroxylases under hypoxia results in metabolic catastrophe and cell death. Oncogene 2009;28:4009-4021.
https://doi.org/10.1038/onc.2009.250 |
| 15 | Patten DA, Lafleur VN, Robitaille GA, Chan DA, Giaccia AJ, Richard DE: Hypoxia-inducible factor-1 activation in nonhypoxic conditions: the essential role of mitochondrial-derived reactive oxygen species. Mol Biol Cell 2010;21:3247-3257.
https://doi.org/10.1091/mbc.e10-01-0025 |
| 16 | Park JO, Rubin SA, Xu YF, Amador-Noguez D, Fan J, Shlomi T, Rabinowitz JD: Metabolite concentrations, fluxes and free energies imply efficient enzyme usage. Nat Chem Biol 2016;12:482-489.
https://doi.org/10.1038/nchembio.2077 |
| 17 | Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB: Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 2015;518:413-416.
https://doi.org/10.1038/nature13981 |
| 18 | Liu PS, Wang H, Li X, Chao T, Teav T, Christen S, Di Conza G, Cheng WC, Chou CH, Vavakova M, Muret C, Debackere K, Mazzone M, Huang HD, Fendt SM, Ivanisevic J, Ho PC: α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol 2017;18:985-994.
https://doi.org/10.1038/ni.3796 |
| 19 | Desideri E, Vegliante R, Ciriolo MR: Mitochondrial dysfunctions in cancer: genetic defects and oncogenic signaling impinging on TCA cycle activity. Cancer Lett 2015;356:217-223.
https://doi.org/10.1016/j.canlet.2014.02.023 |
| 20 | Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E: Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 2005;7:77-85.
https://doi.org/10.1016/j.ccr.2004.11.022 |
| 21 | Laurenti G, Tennant DA: Isocitrate dehydrogenase (IDH), succinate dehydrogenase (SDH), fumarate hydratase (FH): three players for one phenotype in cancer? Biochem Soc Trans 2016;44:1111-1116.
https://doi.org/10.1042/BST20160099 |
| 22 | Eniafe J, Jiang S: The functional roles of TCA cycle metabolites in cancer. Oncogene 2021;40:3351-3363.
https://doi.org/10.1038/s41388-020-01639-8 |
| 23 | Murray AJ: Energy metabolism and the high-altitude environment. Exp Physiol 2016;101:23-27.
https://doi.org/10.1113/EP085317 |
| 24 | Murray AJ, Horscroft JA: Mitochondrial function at extreme high altitude. J Physiol 2016;594:1137-1149.
https://doi.org/10.1113/JP270079 |
| 25 | Lee JW, Ko J, Ju C, Eltzschig HK: Hypoxia signaling in human diseases and therapeutic targets. Exp Mol Med 2019;51:1-13.
https://doi.org/10.1038/s12276-019-0235-1 |
| 26 | Bhattacharyya S, Sinha K, Sil PC: Cytochrome P450s: mechanisms and biological implications in drug metabolism and its interaction with oxidative stress. Curr Drug Metab 2014;15:719-742.
https://doi.org/10.2174/1389200215666141125121659 |
| 27 | Serebrovskaya TV, Nosar VI, Bratus LV, Gavenauskas BL, Mankovska IM: Tissue oxygenation and mitochondrial respiration under different modes of intermittent hypoxia. High Alt Med Biol 2013;14:280-288.
https://doi.org/10.1089/ham.2013.1012 |
| 28 | Yeo EJ:Hypoxia and aging. Exp Mol Med 2019;51:1-15.
https://doi.org/10.1038/s12276-019-0233-3 |
| 29 | MacIntyre NR: Tissue hypoxia: implications for the respiratory clinician. Respir Care 2014;59:1590-1596
https://doi.org/10.4187/respcare.03357 |
| 30 | Vinogradov AD, Gavrikova EV, Grivennikova VG, Zharova TV, Zakharova NV: Catalytic properties of mitochondrial NADH-ubiquinone reductase (Complex I). Biochemistry (Mosc) 1999;64:136-152.
|
| 31 | Vinogradov AD, Grivennikova VG: The mitochondrial complex I: progress in understanding of catalytic properties. IUBMB Life 2001;52:129-134.
https://doi.org/10.1080/15216540152845920 |
| 32 | Zu Y, Shannon RJ, Hirst J: Reversible, electrochemical interconversion of NADH and NAD+ by the catalytic (Ilambda) subcomplex of mitochondrial NADH:ubiquinone oxidoreductase (complex I). J Am Chem Soc 2003;125:6020-6021.
https://doi.org/10.1021/ja0343961 |
| 33 | Murray JL, Mercer SL, Jackson KD: Impact of cytochrome P450 variation on meperidine N-demethylation to the neurotoxic metabolite normeperidine. Xenobiotica 2020;50:209-222.
https://doi.org/10.1080/00498254.2019.1599465 |
| 34 | Rochat B, Amey M, Gillet M, Meyer UA, Baumann P: Identification of three cytochrome P450 isozymes involved in N-demethylation of citalopram enantiomers in human liver microsomes. Pharmacogenetics 1997;7:1-10.
https://doi.org/10.1097/00008571-199702000-00001 |
| 35 | Kobayashi K, Chiba K, Yagi T, Shimada N, Taniguchi T, Horie T, Tani M, Yamamoto T, Ishizaki T, Kuroiwa Y: Identification of cytochrome P450 isoforms involved in citalopram N-demethylation by human liver microsomes. J Pharmacol Exp Ther 1997;280:927-933.
|
| 36 | Poderoso JJ, Helfenberger K, Poderoso C: The effect of nitric oxide on mitochondrial respiration. Nitric Oxide 2019;88:61-72.
https://doi.org/10.1016/j.niox.2019.04.005 |
| 37 | Brown GC: Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta 2001;1504:46-57.
https://doi.org/10.1016/S0005-2728(00)00238-3 |
| 38 | Tirpe AA, Gulei D, Ciortea SM, Crivii C, Berindan-Neagoe I: Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int J Mol Sci 2019;20:6140.
https://doi.org/10.3390/ijms20246140 |
| 39 | Reutov VP, Sorokina EG: NO-synthase and nitrite-reductase components of nitric oxide cycle. Biochemistry (Mosc) 1998;63:874-884.
|
| 40 | Williams D, Venardos KM, Byrne M, Joshi M, Horlock D, Lam NT, Gregorevic P, McGee SL, Kaye DM: Abnormal mitochondrial L-arginine transport contributes to the pathogenesis of heart failure and rexoygenation injury. PLoS One 2014;9:e104643.
https://doi.org/10.1371/journal.pone.0104643 |
| 41 | Zaobornyj T, Ghafourifar P: Strategic localization of heart mitochondrial NOS: a review of the evidence. Am J Physiol Heart Circ Physiol 2012;303:H1283-1293.
https://doi.org/10.1152/ajpheart.00674.2011 |
| 42 | Xu W, Ghosh S, Comhair SA, Asosingh K, Janocha AJ, Mavrakis DA, Bennett CD, Gruca LL, Graham BB, Queisser KA, Kao CC, Wedes SH, Petrich JM, Tuder RM, Kalhan SC, Erzurum SC: Increased mitochondrial arginine metabolism supports bioenergetics in asthma. J Clin Invest 2016;126:2465-2481.
https://doi.org/10.1172/JCI82925 |
| 43 | Zuo S, Zhang Y, Wang Z, Wang J: Mitochondria-Targeted Mesoporous Titanium Dioxide Nanoplatform for Synergistic Nitric Oxide Gas-Sonodynamic Therapy of Breast Cancer. Int J Nanomedicine 2022;17:989-1002.
https://doi.org/10.2147/IJN.S348618 |
| 44 | Kondrashova MN, Doliba NM: Polarographic observation of substrate-level phosphorylation and its stimulation by acetylcholine. FEBS Lett 1989;243:153-155.
https://doi.org/10.1016/0014-5793(89)80119-X |
| 45 | Wisniewski JA, Moody DE, Hammock BD, Shull LR: Interlobular distribution of hepatic xenobiotic-metabolizing enzyme activities in cattle, goats and sheep. J Anim Sci 1987;64:210-215.
https://doi.org/10.2527/jas1987.641210x |
| 46 | Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-254.
https://doi.org/10.1016/0003-2697(76)90527-3 |
| 47 | Chance B, Williams GR: Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem 1955;217:409-427.
https://doi.org/10.1016/S0021-9258(19)57191-5 |
| 48 | Chance B: Reaction of oxygen with the respiratory chain in cells and tissues. J Gen Physiol 1965;49:163-195.
https://doi.org/10.1085/jgp.49.1.163 |
| 49 | Chance B, Williams GR: Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 1955;217:383-393.
https://doi.org/10.1016/S0021-9258(19)57189-7 |
| 50 | Karuzina II, Archakov AI: Estimation of the microsomal fraction of the liver and characteristic of its oxidative systems. Modern methods in biochemistry. Ed. V.I. Orekhovich. Moscow, Medicine, 1977:49-52.
|
| 51 | Matsubara T, Touchi A, Tochino Y: Hepatic aminopyrine N-demethylase system: further studies of assay procedure. Jpn J Pharmacol 1977;27:127-136.
https://doi.org/10.1254/jjp.27.127 |
| 52 | Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR: Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1982;126:131-138.
https://doi.org/10.1016/0003-2697(82)90118-X |
| 53 | Brown GC, Cooper CE: Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 1994;356:295-298.
https://doi.org/10.1016/0014-5793(94)01290-3 |
| 54 | Kolb VG, Kamyshnikov VS: A guide on clinical chemistry. Minsk: Republic of Belarus, 1982, 336. [In Russian]
|
| 55 | Faber J, Menashe M, Bachrach U, Desser H: Formation of putrescine and acetylspermidine from spermidine by cultured human lymphocytes. FEBS Lett 1980;121:165-168.
https://doi.org/10.1016/0014-5793(80)81289-0 |
| 56 | Kamyshnikov VS: A reference book on the clinic and biochemical research and laboratory diagnostics. Moscow, MEDpress-inform, 2004, 911 p. [In Russian]
|
| 57 | Levine RL, Williams JA, Stadtman ER, Shacter E: Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 1994;233:346-357.
https://doi.org/10.1016/S0076-6879(94)33040-9 |
| 58 | Dubinina EE, Burmistrov SO, Khodov DA, Porotov IG: Okislitel'naia modifikatsiia belkov syvorotki krovi cheloveka, metod ee opredeleniia [Oxidative modification of human serum proteins. A method of determining it]. Vopr Med Khim 1995;41:24-26. [In Russian]
|
| 59 | Kostiuk VA, Potapovich AI, Kovaleva ZhV: Prostoĭ i chuvstvitel'nyĭ metod opredeleniia aktivnosti superoksid-dismutazy, osnovannyĭ na reaktsii okisleniia kvertsetina [A simple and sensitive method of determination of superoxide dismutase activity based on the reaction of quercetin oxidation]. Vopr Med Khim 1990;36:88-91. [In Russian]
|
| 60 | Koroliuk MA, Ivanova LI, Maĭorova IG, Tokarev VE: Metod opredeleniia aktivnosti katalazy [A method of determining catalase activity]. Lab Delo 1988;1:16-19. [In Russian]
|
| 61 | Glatzle D, Vuilleumier JP, Weber F, Decker K: Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientia 1974;30:665-667.
https://doi.org/10.1007/BF01921531 |
| 62 | Moin VM: Prostoĭ i spetsificheskiĭ metod opredeleniia aktivnosti glutationperoksidazy v éritrotsitakh [A simple and specific method for determining glutathione peroxidase activity in erythrocytes]. Lab Delo 1986; 12:724-727. [In Russian]
|
| 63 | Reitman S, Frankel S: A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 1957;28:56-63.
https://doi.org/10.1093/ajcp/28.1.56 |
| 64 | Eschenko ND, Volski GG: Determination of succinic acid level and succinate dehydrogenase activity. In: Methods of biochemical study. Leningrad: National Leningrad University, 1982, 352. [In Russian ]
|
| 65 | Shutskiĭ IV: Metod opredeleniia atsetilkholina v malykh kolichestvakh krovi [A method for determining acetylcholine in small amounts of blood]. Lab Delo 1967;7:407-408. [In Russian]
|
| 66 | Menshikov VV: Laboratory methods of research in the clinic practice. Moscow, Medicine, 1987, p. 225-227. [In Russian]
|
| 67 | Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM: A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88-95.
https://doi.org/10.1016/0006-2952(61)90145-9 |
| 68 | Osinskaya OV: Investigation of the metabolism of epinephrine and norepinephrine in tissues of the animal's organism. Biochemistry 1957;2:537-553. [In Russian]
|
| 69 | Laverty R, Taylor KM: The fluorometric assay of catecholamines and related compounds: improvements and extensions to the hydroxyindole technique. Anal Biochem 1968;22:269-279.
https://doi.org/10.1016/0003-2697(68)90316-3 |
| 70 | Jacobowitz DM, Richardson JS: Method for the rapid determination of norepinephrine, dopamine, and serotonin in the same brain region. Pharmacol Biochem Behav 1978;8:515-519.
https://doi.org/10.1016/0091-3057(78)90380-5 |
| 71 | Zar JH: Biostatistical Analysis. 4th ed., Prentice Hall Inc., New Jersey, 1999.
|
| 72 | Rochette L, Lorin J, Zeller M, Guilland JC, Lorgis L, Cottin Y, Vergely C: Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol Ther 2013;140:239-257.
https://doi.org/10.1016/j.pharmthera.2013.07.004 |
| 73 | Merelli A, Repetto M, Lazarowski A, Auzmendi J: Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J Alzheimers Dis 2021;82:S109-S126.
https://doi.org/10.3233/JAD-201074 |
| 74 | Wang X, Liu K, Li B, Li Y, Ye K, Qi J, Wang Y: Macrophages Aggravate Hypoxia-Induced Cardiac Microvascular Endothelial Cell Injury via Peroxynitrite: Protection by Tongxinluo. Cell Commun Adhes 2015;22:39-47.
https://doi.org/10.3109/15419061.2016.1155565 |
| 75 | Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Däbritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, O'Neill LA: Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016;167:457-470.e13.
https://doi.org/10.1016/j.cell.2016.08.064 |
| 76 | Porcelli AM, Ghelli A, Ceccarelli C, Lang M, Cenacchi G, Capristo M, Pennisi LF, Morra I, Ciccarelli E, Melcarne A, Bartoletti-Stella A, Salfi N, Tallini G, Martinuzzi A, Carelli V, Attimonelli M, Rugolo M, Romeo G, Gasparre G: The genetic and metabolic signature of oncocytic transformation implicates HIF1alpha destabilization. Hum Mol Genet 2010;19:1019-1032.
https://doi.org/10.1093/hmg/ddp566 |
| 77 | Brocherie F, Timon R: Editorial: Long-term effects of hypoxic conditioning on sports performance, health and well-being. Front Physiol 2022;13:1112754.
https://doi.org/10.3389/fphys.2022.1112754 |
| 78 | Brocherie F, Millet GP: Hypoxic exercise as an effective nonpharmacological therapeutic intervention. Exp Mol Med 2020;52:529-530.
https://doi.org/10.1038/s12276-020-0400-6 |
| 79 | Navarrete-Opazo A, Mitchell GS: Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 2014;307:R1181-1197 .
https://doi.org/10.1152/ajpregu.00208.2014 |
| 80 | Yuan H, Liu J, Gu Y, Ji X, Nan G: Intermittent hypoxia conditioning as a potential prevention and treatment strategy for ischemic stroke: Current evidence and future directions. Front Neurosci 2022;16:1067411.
https://doi.org/10.3389/fnins.2022.1067411 |
| 81 | Gusakova SV, Smagliy LV, Birulina YG, Kovalev IV, Nosarev V, Petrova IV, Reutov VP: [Molecular Mechanisms of Action of Gas Transmitters NO, CO and H2S in Smooth Muscle Cells and Effect of NO-generating Compounds (Nitrates and Nitrites) on Average Life Expectancy]. Usp Fiziol Nauk 2017;48:24-52. [In Russian]
|
| 82 | Boughaleb H, Lobysheva I, Dei Zotti F, Balligand JL, Montiel V: Biological Assessment of the NO-Dependent Endothelial Function. Molecules 2022;27:7921.
https://doi.org/10.3390/molecules27227921 |
| 83 | Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, Trimarco V: Arginine and Endothelial Function. Biomedicines 2020;8:277.
https://doi.org/10.3390/biomedicines8080277 |
| 84 | Szefel J, Danielak A, Kruszewski WJ: Metabolic pathways of L-arginine and therapeutic consequences in tumors. Adv Med Sci 2019;64:104-110.
https://doi.org/10.1016/j.advms.2018.08.018 |
| 85 | Ratnikova LA, Chistiakov VV, Iaguzhinskiĭ LS: Reguliatornye vzaimodeĭstviia dykhatel'noĭ tsepi mitokhondriĭ i okislitel'noĭ sistemy éndoplazmaticheskogo retikuluma [Regulatory interaction between the respiratory chain of mitochondria and the oxidative system of the endoplasmic reticulum]. Biokhimiia 1978;43:1809-1815. [In Russian]
|
| 86 | Fan Y, Simmen T: Mechanistic Connections between Endoplasmic Reticulum (ER) Redox Control and Mitochondrial Metabolism. Cells 2019;8:1071.
https://doi.org/10.3390/cells8091071 |
| 87 | Picard M, McEwen BS, Epel ES, Sandi C: An energetic view of stress: Focus on mitochondria. Front Neuroendocrinol 2018;49:72-85.
https://doi.org/10.1016/j.yfrne.2018.01.001 |
| 88 | Palkovits M: Catecholamines and stress. Ideggyogy Sz 2014;67:116-120.
|
| 89 | Manukhina EB, Mashina SYu, Smirin BV, Lyamina NP, Senchikhin VN, Vanin AF, Malyshev IYu: Role of nitric oxide in adaptation to hypoxia and adaptive defense. Physiol Res 2000;49:89-97.
|
| 90 | Sprick JD, Mallet RT, Przyklenk K, Rickards CA: Ischaemic and hypoxic conditioning: potential for protection of vital organs. Exp Physiol 2019;104:278-294.
https://doi.org/10.1113/EP087122 |
| 91 | Sprick JD, Rickards CA: Combining remote ischemic preconditioning and aerobic exercise: a novel adaptation of blood flow restriction exercise. Am J Physiol Regul Integr Comp Physiol 2017;313:497-506.
https://doi.org/10.1152/ajpregu.00111.2017 |
| 92 | Yang Y, Gao C, Yang T, Sha Y, Cai Y, Wang X, Yang Q, Liu C, Wang B, Zhao S: Vascular characteristics and expression of hypoxia genes in Tibetan pigs' hearts. Vet Med Sci 2022;8:177-186.
https://doi.org/10.1002/vms3.639 |
| 93 | Zheng WS, He YX, Cui CY, Ouzhu L, Deji Q, Peng Y, Bai CJ, Duoji Z, Gongga L, Bian B, Baima K, Pan YY, Qu L, Kang M, Ciren Y, Baima Y, Guo W, Yang L, Zhang H, Zhang XM, Guo YB, Xu SH, Chen H, Zhao SG, Cai Y, Liu SM, Wu TY, Qi XB, Su B: EP300 contributes to high-altitude adaptation in Tibetans by regulating nitric oxide production. Zool Res 2017;38:163-170.
|
| 94 | Greijer AE, van der Wall E: The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol 2004;57:1009-1014.
https://doi.org/10.1136/jcp.2003.015032 |
| 95 | Zhao S, Wu W, Lin X, Shen M, Yang Z, Yu S, Luo Y: Protective effects of dexmedetomidine in vital organ injury: crucial roles of autophagy. Cell Mol Biol Lett 2022;27:34.
https://doi.org/10.1186/s11658-022-00335-7 |
| 96 | Carraro F, Pucci A, Pellegrini M, Pelicci PG, Baldari CT, Naldini A: p66Shc is involved in promoting HIF-1alpha accumulation and cell death in hypoxic T cells. J Cell Physiol 2007;211:439-447.
https://doi.org/10.1002/jcp.20951 |