Elucidation of the Role of L-Arginine and Nω-Nitro-L-Arginine in the Treatment of Rats with Different Levels of Hypoxic Tolerance and Exposure to Lead Nitrate
bDepartment of Ecology, Geography and Nature Management, T.H. Shevchenko National University “Chernihiv Colehium”, 53 Hetmana Polubotka Str., Chernihiv, 14013, Ukraine,
cNicolausCopernicusUniversity in Toruń, Collegium Medicum in Bydgoszcz, Department of Medical Biology and Biochemistry, Department of Ecology and Environmental Protection, M. Skłodowska-Curie St. 9, PL 85-094 Bydgoszcz, Poland,
dUniversity of Zielona Góra, Faculty of Biological Sciences, Institute of Biological Sciences, Department of Biotechnology, Prof. Z. Szafran St. 1, PL 65-516 Zielona Góra, Poland
Keywords
Abstract
Background/Aims:
Individual resistance to hypoxia is an important feature of the physiological profile of an organism, particularly in relation to lead-induced toxicity.Methods:
Our study focused on evaluating parameters of mitochondrial oxygen consumption, microsomal oxidation, intensity of lipoperoxidation processes and antioxidant defences in the liver of rats with low (LR) and high (HR) resistance to hypoxia to elucidate the mechanisms of action of L-arginine and the NO synthase inhibitor L-NNA before or after exposure to lead nitrate.Results:
Our study suggests that the redistribution of oxygen-dependent processes towards mitochondrial processes under the influence of the nitric oxide precursor amino acid L-arginine is an important mechanism for maintaining mitochondrial respiratory chain function during per os lead nitrate exposure (3.6 mg lead nitrate/kg bw per day for 30 days). Animals were given L-arginine at a dose of 600 mg/kg bw (i.p., 30 min) before and after exposure to lead nitrate or the NO synthase inhibitor Nω-nitro-L-arginine (L-NNA) at a dose of 35 mg/kg bw (i.p., 30 min) before and after exposure to lead nitrate. Our experiments demonstrated the efficacy of using lead nitrate to simulate lead-related toxic processes via Pb levels in liver tissue; we demonstrated significantly reduced levels of nitrites and nitrates, i.e. stable metabolites of the nitric oxide system, in both LR and HR animals. The effect of the amino acid L-arginine stabilised the negative effects of lead nitrate exposure in both groups of LR and HR rats. We observed the efficiency of mitochondrial energy supply processes and showed a greater vulnerability of NADH-dependent oxidation during lead nitrate exposure in the liver of HR rats.Conclusion:
L-arginine initiated the processes of oxidation of NADH-dependent substrates in the LR group, whereas in the HR group this directionality of processes was more effective when the role of the nitric oxide system was reduced (use of L-NNA). Our study of key antioxidant enzyme activities in rat liver tissue during lead nitrate exposure revealed changes in the catalase-peroxidase activity ratio. We found different activities of antioxidant enzymes in the liver tissue of rats treated with lead nitrate and L-arginine or L-NNA, with a significant increase in GPx activity in the LR group when L-arginine was administered both before and after exposure to lead nitrate.Introduction
Many studies have shown that lead can cause serious health effects in both animals and humans (Assi et al., 2016; Karp, 2023). As a widespread environmental contaminant, lead causes a wide range of adverse health effects, including neurological disorders, developmental delay, cardiovascular disease, kidney damage, and reproductive disorders, as convincingly demonstrated in a number of studies (Navas-Acien et al., 2007; Rastogi, 2008; Mason et al., 2014; Jan et al., 2015). In addition, lead exposure is particularly harmful to children, as it can impair cognitive development, lower the IQ, and lead to behavioural problems that may persist into adulthood (Ramírez Ortega et al., 2021). Therefore, lead research is important to understand the mechanisms of lead toxicity, identify vulnerable populations, and inform effective prevention and intervention strategies. In addition, understanding the effects of lead on animal health is critical for protecting ecosystems and biodiversity by assessing the cascading effects on wildlife populations, leading to declines, reproductive failure, and disruptions in food chains (Briffa et al., 2020). By studying the effects of lead on both animals and humans, researchers will be able to develop integrated approaches to reduce its toxic effects, protect public health, and preserve environmental quality.
The mechanisms of action of lead (Pb) can lead to a wide range of toxic effects in the body, highlighting the importance of research to understand these processes in order to protect human and environmental health (Balali-Mood et al., 2021). Research suggests that lead causes damage to cellular structures and metabolic disorders by interacting with proteins containing sulphhydryl (-SH) groups, leading to protein denaturation and altered function (Dabrowska-Bouta et al., 1996; Jurczuk et al., 2006). This can lead to enzyme inhibition. Experiments show that lead can inhibit the activity of a number of enzymes, such as those involved in heme synthesis, amino acid synthesis, or detoxification processes, leading to disturbances in metabolic processes and cellular functions (Balali-Mood et al., 2021; Virgolini and Aschner, 2021).
Lead can interfere with mitochondrial function, disrupting cellular respiration processes and leading to the generation of excess reactive oxygen species (ROS), which can cause damage to DNA, proteins, and lipids, contributing to increased inflammatory processes and exacerbating oxidative stress (Bandaru et al., 2023). Numerous studies have suggested that oxidative stress with subsequent lipid peroxidation is one mechanism of lead toxicity (Lee et al., 2019). The next set of experiments focused on analysing the redox status in the liver of rats exposed to lead nitrate. Significant increases in both lipid hydroperoxides and TBARS levels were observed in the liver of lead-exposed rats. Oxidative stress is thought to be involved in lead-induced toxicity by inducing the generation of ROS, including hydroperoxides, singlet oxygen, hydrogen peroxide and superoxide, and by reducing the antioxidant defence system of cells (Sharma et al., 2014). Lead causes oxidative stress by inducing the generation of reactive oxygen species, reducing the antioxidant defence system of cells by depleting glutathione, inhibiting sulfhydryl-dependent enzymes, interfering with some essential metals required for antioxidant enzyme activities, and/or increasing the susceptibility of cells to oxidative attack by altering membrane integrity and fatty acid composition (Hsu and Guo, 2002).
Lead can disrupt signalling between cells by altering signalling pathways, cell receptors, and gene expression (Gillis et al., 2012; Metryka et al., 2018). Lead can affect the activity of several signalling pathways, including the MAPK/ERK pathway, the JAK/STAT pathway, and the NF-κB pathway (Renu et al., 2021). Studies have shown that the mechanism of action of lead involves inhibition or enhancement of the phosphorylation of signalling proteins, leading to changes in signalling in the cell. For example, lead can inhibit MAPK kinase activity, resulting in the disruption of growth factor and cytokine-stimulated signalling (Teschke, 2022).
Studies have also shown that lead can affect the structure, localisation, and function of membrane receptors, such as receptors for growth factors and hormones or gene expression by interacting with regulatory elements within gene promoters, modifying chromatin, or interfering with transcriptional mechanisms as a nuclear receptor agonist. Such effects activate neurotransmitters through internalisation of membrane receptors, leading to reduced cell surface receptor availability and signal transduction efficiency. Lead can affect the transcription of genes associated with stress and toxic system responses (Wagner et al., 2017; Park et al., 2024).
Many studies highlight the long-term toxic effects of lead poisoning, as this element accumulates in bones and teeth, replacing calcium and disrupting mineralisation processes (Rodríguez and Mandalunis, 2018). This can lead to bone damage, increased risk of fractures, and excretion of lead from bones into the bloodstream during a period of increased bone resorption (Pounds et al., 1991). Nerve conduction, muscle contraction, and protein synthesis are all affected by lead toxicity, which is linked to the homeostasis of calcium, magnesium, zinc, and other trace element ions, resulting in disruption of many physiological processes (Balali-Mood et al., 2021). These mechanisms of lead action can be complex and involve interactions at multiple levels, both within the single cell and in intercellular signalling pathways. These molecular changes have consequences for a variety of physiological processes, leading to a range of toxic effects on the body (Sakai, 2000).
In recent decades, researchers have been able to elucidate the role of the amino acid L-arginine in supporting repair and regeneration processes in cells, which is important in the case of damage caused by toxic agents (El-Sheikh and Khalil, 2011; Li et al., 2022). However, the subject of the multiple effects of L-arginine as a precursor of nitric oxide, which plays an important role in the functioning of the mammalian organism, is still topical. Research on L-arginine may therefore shed light on its effect on the body’s ability to repair itself (Kurhaluk, 2024). Research on the amino acid L-arginine is also important for understanding the body’s defence mechanisms against toxic agents and may provide clues for prevention and therapy strategies for poisoning by various substances, as has been convincingly shown in various animal models (Szlas et al., 2022; Kurhaluk, 2023a).
Previously, our research provided a deeper understanding of the role of the amino acid L-arginine in regulating oxygen-dependent processes in different stress conditions and in different animal models (Kurhaluk, 2023b). We investigated how individual variations in physiological resistance to hypoxia may influence this interaction through such mechanisms as detoxification or neutralisation of ROS (Kurhaluk et al., 2021; Kurhaluk, 2023a, b, c). In this context, our studies investigated the relationship between hypoxia resistance and the response to lead poisoning (Tkachenko et al., 2007; Tkachenko and Kurhalyuk, 2011). We sought to determine whether rats with different levels of hypoxia resistance have different responses to lead poisoning and whether L-arginine influences these differences. It is conceivable that individuals with naturally higher hypoxia resistance may have an enhanced ability to neutralise the toxic effects of lead.
Although some research considers individual resistance to hypoxia as a characteristic of the physiological profile of an organism, there are still no studies in this area. It is therefore important and intriguing, particularly in relation to heavy metal poisoning, among which lead occupies a prominent place due to its toxicity. It is known that the efficiency of NO storage is genetically determined and appears to be related to the inherited capacity for NO synthesis (Kurhaliuk, 2001). Individual resistance to hypoxia involves unique responses in the mitochondrial respiratory chain function, ion transport, mitochondrial enzyme functional properties, and energy metabolism (Kurhaluk et al., 2019; Belosludtsev et al., 2020; Dzhalilova et al., 2018, 2023a, b; Kosyreva et al., 2023; Kurhaluk, 2023a). It is particularly important in the biotransformation of xenobiotics and the drug metabolism system (Bayanov and Brunt, 1999) in the formation of individual cellular resistance to oxygen deprivation. Constitutional resistance to hypoxia can serve as an important criterion for an individualised approach to the pharmacotherapy of hypoxic states and diseases and for the prognosis and prevention of early and distant complications of irrational pharmacotherapy (Lukyanova et al., 2020; Dzhalilova et al., 2023; Kurhaluk, 2023a, b).
The aims of this study are i) to investigate different effects of L-arginine and the NO synthase inhibitor Nω-nitro-L-arginine (L-NNA) on lead-induced toxicity in rats with high and low hypoxic resistance in terms of mitochondrial respiration, microsomal oxidation processes, oxidative stress, and antioxidant defence; ii) to elucidate how variations in mitochondrial function contribute to oxidative stress levels and subsequent antioxidant responses in lead poisoning in rats with different individual hypoxic resistance; iii) to use statistical analysis to assess the correlations between parameters of mitochondrial oxidative phosphorylation, oxidative stress markers, and antioxidant enzyme activities to gain insight into the underlying mechanisms of lead action and the correction of these processes by L-arginine and L-NNA; iv) to determine whether L-arginine and L-NNA act primarily by preventing lead-induced toxicity (pre-exposure effect) or by attenuating the toxic effects of lead (post-exposure effect). Importantly, research on the metabolism of lead and the amino acid L-arginine and L-NNA as inhibitors of NO synthase should consider the role of different tissue-dependent processes (mitochondrial respiration, microsomal oxidation, lipid peroxidation), as interactions between them may be crucial for understanding the mechanisms of lead toxicity and the potential protective effects of L-arginine and L-NNA.
Materials and Methods
Ethical statement
The experiments were conducted in accordance with the guidelines of the Council of the European Union, current legislation in Poland and Ukraine, and the recommendations of the Ethics Committee. They were approved by the Ethics Committee of the T.H. Shevchenko National University “Chernihiv Colehium” (28/09/2020). The experiment was conducted in accordance with both Directive 2010/63/EU on the protection of animals used for scientific purposes and the Polish Act of 15 January 2015 on the protection of animals used for scientific or educational purposes (Journal of Laws of 26 February 2015, item 266).
Experimental animals
Male white rats (180-220 g) were used in the study. The rats were maintained at a constant temperature of 23 ± 2oC with a 12:12 h light/dark cycle and 40-50% relative humidity, with unrestricted access to food and water. The animals (n = 6 per group) had free access to food and water throughout the experiments. All manipulations with the animals were carried out in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, 2006), and every effort was made to minimise animal distress and suffering.
Determination of resistance to hypobaric hypoxia
After 15 days of adaptive feeding, the animals were placed once in a ventilated pressurised chamber at an ‘altitude’ of 11, 000 m (Dzhalilova et al., 2019a, b, 2023a,b). The time to the first sign of the characteristic hyperventilation response (‘gasp time’) was recorded using a stopwatch. Different measures of the gasp time are used to separate animals according to hypoxia resistance, but the gasp time of tolerant animals should be at least three times higher than that of susceptible animals (Mironova et al., 2010; Kurhaluk et al., 2019; Dzhalilova et al., 2019a, b, 2023b). The animals were divided into three groups based on their panting time: ‘low resistant’ (< 80 s), ‘normal’ (80-240 s), and ‘high resistant’ (> 240 s). The ‘normal’ group was excluded from further experiments. Two groups of rats were used for further experiments: rats with low resistance and rats with high resistance to hypoxia.
Experimental groups
One month after the hypoxia resistance test, the rats were randomly divided into six groups. Group I with low resistance (n = 6) and high resistance to hypoxia (n = 6) served as a control and received daily injections of sterile normal saline for 30 days. Group II (Pb group) with low resistance (n = 6) and high resistance to hypoxia (n = 6) received 3.6 mg lead nitrate/kg b.w. daily by oral gavage. Group III (L-arginine and Pb group) with low resistance (n = 6) and high resistance to hypoxia (n = 6) also received 3.6 mg lead nitrate/kg b.w. daily for 30 days, during which time the animals were intraperitoneally (i.p.) injected with L-arginine at a dose of 600 mg/kg b.w. prior to 30-min lead nitrate treatment. Group IV (L-NNA and Pb group) was treated as group III except that it received Nω-nitro-L-arginine injection (35 mg/kg b.w. L-NNA) before 30-min lead nitrate treatment. Group V (Pb group and L-arginine) with low resistance (n = 6) and high resistance to hypoxia (n = 6) also received 3.6 mg lead nitrate/kg b.w. daily for 30 days, during which the animals were injected intraperitoneally (i.p.) with L-arginine at a dose of 600 mg/kg b.w. after 30-min lead nitrate treatment. Group VI (Pb group and L-NNA) was treated as group V except that it received Nω-nitro-L-arginine injection (35 mg/kg b.w. L-NNA) after 30-min lead nitrate treatment. The doses of L-arginine and L-NNA were chosen on the basis of our previous studies (Kurhaliuk, 2001; Tkachenko et al., 2007; Tkachenko and Kurhalyuk, 2011).
On day 31 after the start of the experiment, all rats were euthanised by intraperitoneal injection of a lethal dose of sodium pentobarbital (Morbital, Biowet, Pulawy; 200 mg/kg b.w.).
Samples
Livers were removed from the rats immediately after decapitation. One animal was used for each preparation. The isolated liver was excised, weighed, and washed in ice-cold isolation buffer containing 2 mM K2CO3, 10 mM HEPES, and 1 mM EGTA (pH 7.2). The minced tissue was rinsed to remove blood with cold isolation buffer and homogenised on ice in a Potter-Elvehjem glass homogeniser using a Teflon motorised pestle. The suspension was then centrifuged at 600g for 5 minutes at 0◦C. The mitochondrial fraction was obtained by centrifugation of the supernatant at 8, 000g for 12 minutes at 0◦C. The mitochondria were resuspended in isolation buffer. The mitochondrial suspension (4-6 mg protein/mL) was kept on ice before the experiment (Kondrashova and Doliba, 1989). A detailed description of these isolation and analysis techniques can be found in our previous work (Kurhaluk et al., 2023c).
The liver microsomal fraction was isolated according to the methods proposed by Wisniewski et al. (1987). A detailed description of the reference to these isolation and analysis techniques is given in Kurhaluk et al. (2023c). The protein concentration was determined with the Bradford assay (1976) using bovine serum albumin as a standard.
Quantitative analyses of lead
Quantitative analyses of Pb in the rat liver tissue were performed using the ICP-MS technique. The instrumentation consisted of an Agilent 7500ce ISP-MS apparatus equipped with a micro-mist nebuliser, a Peltier-cooled double-pass spray chamber, and a peristaltic pump. Argon 5.0 (99.999% purity) was used as the carrier gas. The facility included a shielding torch system to minimise secondary discharge, off-axis ion lenses, and a reaction/collision chamber using hydrogen 6.0 and helium 6.0 (99.9999% purity) to eliminate interference. The vacuum system included both a rotary pump and a turbomolecular pump. Pb concentrations (expressed as μg·g-1 dry weight) were determined using the calibration curve method with three replicates for each sample. Further methodological details can be found in a previous publication (Tkachenko et al., 2022).
Assessment of mitochondrial respiration using the oxygraphic method
Mitochondrial respiratory function was measured in a multichannel chamber using a Clark-type electrode in accordance with the polarographic method proposed by Chance and Williams (1955a,b). Mitochondria were placed in the respiratory chamber containing a total volume of 1.0 ml of incubation medium. The medium contained 120 mM KCl, 2 mM K2CO3, 2 mM KH2PO4, and 10 mM HEPES. Potassium hydroxide (1.0 N) was used to adjust the pH of the medium to 7.20 at 26 oC. Succinate (SC, 0.35 mM final concentration) and α-ketoglutarate (KGL, 1 mM) were used as oxidative substrates. Glutamate (3 mM final concentration) and malate (2.5 mM) and mixtures of glutamate (3 mM) and pyruvate (3 mM) were used as donors of α-ketoglutarate by aminotransferase reaction. ADP (phosphate acceptor) was administered at a concentration of 0.2 mM.
The following parameters of mitochondrial oxygen consumption were measured: State 2 (oxygen consumption before addition of ADP), State 3 (oxygen consumption stimulated by ADP), State 4 (oxygen consumption after cessation of ADP phosphorylation), the respiratory control ratio (RCR) described by Chance (ratio of State 3 to State 4), the ADP/O ratio (ratio of nanomoles of ADP phosphorylated to nanomoles of oxygen consumed during State 3), and the rate of phosphorylation (Vph). The RCR and the ADP/O ratio were calculated as in Chance and Williams (1955a,b) and Chance (1956).
Biochemical assays
- The assessment of diene conjugates (DK) in the sample was performed using the method introduced by Kamyshnikov (2004) in a heptane-isopropanol mixture. The amount of diene conjugates was then determined spectrophotometrically at 233 nm and expressed per mg protein.
- TBARS assay for lipid peroxidation: The level of lipid peroxidation was determined by measuring the concentration of 2-thiobarbituric acid reactive substances (TBARS) using the method proposed by Buege and Aust for the determination of the malonic dialdehyde (MDA) concentration.
- The protein carbonyl derivative assay was performed to assess the levels of oxidatively modified proteins (OMP). The resulting carbonyl derivatives of amino acids were reacted with 2, 4-dinitrophenylhydrazine (DNFH) according to the method described by Reznick and Packer (1994). Carbonyl groups were quantified spectrophotometrically at 370 nm and 430 nm (for aldehydic derivatives, AD OMP and ketonic derivatives, KD OMP) and the results were expressed as nmol per mg of protein.
- Superoxide dismutase (SOD) activity was determined by a method described by Kostiuk et al. (1990). This involved measuring the ability of SOD to dismutate superoxide generated during the auto-oxidation of quercetin in an alkaline medium (pH 10.0). The resulting activity was expressed as units of SOD per mg of protein.
- Catalase activity (CAT) was determined by measuring the reduction of H2O2 in the reaction mixture using a spectrophotometer at a wavelength of 410 nm according to the method described by Koroliuk and co-workers (1988). One unit of catalase activity was defined as the amount of enzyme required to reduce 1 μmol H2O2 per minute per mg of protein.
- Glutathione reductase (GR) activity was measured in the samples according to the method described by Glatzle and co-workers (1967) with some modifications. GR activity was expressed as nmol of NADPH2 per minute per mg of protein.
- Glutathione peroxidase (GPx) activity was determined by observing the non-enzymatic consumption of GSH, the reacting substrate, at an absorbance of 412 nm after incubation with 5, 5-dithiobis-2-nitrobenzoic acid (DTNB) according to the Moin assay (1986). The resulting GPx activity was expressed as μmol GSH per minute per mg protein.
- The total antioxidant capacity (TAC) of the samples was assessed by quantifying the level of 2-thiobarbituric acid reactive substances (TBARS) after oxidation of Tween-80 (Galaktionova et al., 1998). This level was measured spectrophotometrically at 532 nm. The inhibition of the Fe2+/ascorbate-induced oxidation of Tween 80 resulted in a reduction in TBARS levels. The TAC content in the sample (%) was calculated relative to the absorbance of the blank sample.
- Nitric oxide system assays: Assays of the nitric oxide system were performed to assess its activity. The concentration of the stable nitrite anion metabolite (NO2–) was measured spectrophotometrically according to the protocol established by Green et al. (1982). The results were expressed in picomoles per milligram protein. In addition, nitrate ions (NO3–) were determined with the method described by Brown and Cooper (1994) and expressed as nanomoles per milligram of protein.
- Aminopyrine N-demethylase activity assay: The assay for aminopyrine N-demethylase activity involved measurement of the enzymatic activity of cytochrome P450-dependent aminopyrine N-demethylase as described by Karuzina and Archakov (1977). This method is based on the formaldehyde reaction facilitated by the Nash reagent as described by Matsubara et al. (1977). In this process, cytochrome P450, NADPH, and oxygen are necessary components. The reaction mixture contained 3 mM NADPH2, 0.1 M Tris-HCl, 0.25 M Tris, 8 mM aminopyrine, and 5 mM magnesium chloride. After incubation, hepatic microsomal activity was quantified by measuring the nanomoles of formaldehyde produced per minute per milligram of microsomal protein. Study design scheme was shown in Fig.1.
Fig. 1: Study design scheme.
Statistical analysis
Basic statistical ANOVA analyses were performed using the Statistica 13.3 package (TIBCO Inc., USA). Data were tested for homogeneity of variance using Levene’s test for equality of error variances. Normality was tested using the Kolmogorov-Smirnov test. The results are expressed as mean ± S.E.M. Significant differences between means were measured using the test at p < 0.05. Non-normally distributed data were logarithmically transformed. ANOVA tests with 95% confidence intervals (α = 0.05) and Tukey post-hoc tests were used to determine the significance of differences between the groups of the animals analysed (Zar, 1999).
Results
Pb content
In our study, we used oral lead nitrate as a model of lead-induced toxicity. The results of this series of experiments on individual physiological responses (high and low resistance to hypoxia, HR and LR) during lead-induced exposure and the correction of this toxicity by the nitric oxide synthesis precursor, the amino acid L-arginine, and the nitric oxide synthesis inhibitor L-NNA, administered before (30 min) and after (30 min) lead administration over the 30-day experimental period are shown in Fig. 2.
Our model of lead poisoning showed a significant twofold increase in lead levels in the liver tissue of the animals, compared to the control. Notably, this accumulation of lead in the liver was higher in the HR animals, compared to the LR animals. The other significant findings of lead nitrate effects in our experiments were related to the statistically significant reduction in liver lead levels in the LR animals when L-arginine was administered prior to the lead exposure and when L-NNA was administered at 30 minutes after the lead nitrate exposure, compared to the group of HR rats treated with lead nitrate alone.
In our study, we used a model of lead-induced toxicity with oral administration of lead nitrate. Our experiments showed a significant increase in lead levels in the liver tissue of the animals, compared to the control group, suggesting the efficacy of the model in simulating lead-induced toxic processes. We also observed differences in lead accumulation between the HR and LR animals, suggesting different sensitivities of the rats to lead toxicity. The administration of L-arginine before the exposure to lead nitrate and L-NNA after the exposure significantly reduced liver lead levels in these groups of animals, suggesting the potential efficacy of these substances in preventing lead-induced toxicity.
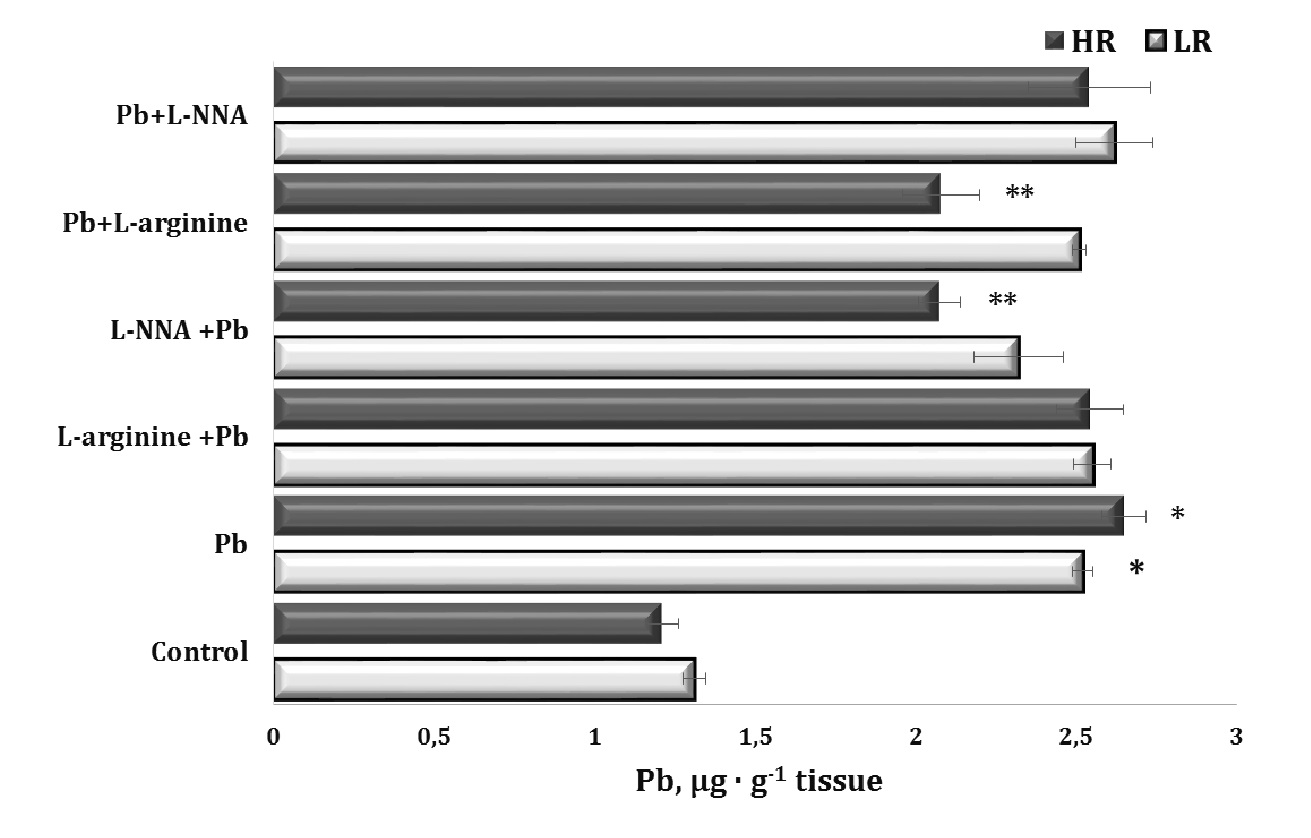
Fig. 2: Lead levels in liver tissue of rats with low (LR) and high (HR) hypoxia resistance exposed to lead nitrate for 30 days and treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before and after exposure to lead nitrate. Data are expressed as mean ± S.E.M. (n = 6). * Significant differences between Pb group and untreated control (p<0.05); ** Significant differences between Pb and L-arginine, Pb and L-NNA treated groups (p<0.05).
Nitrite and nitrate levels
Since lead-induced toxicity can lead to dysfunction of the nitric oxide system, its reactivity and ability to interact with other regulatory factors and the levels of stable metabolites of its metabolism, such as nitrites and nitrates, are important elements in the assessment of NO-related metabolic changes. In our study, lead nitrate significantly reduced the nitrite and nitrate-dependent components of the stable metabolites of the NO-related system (Fig. 3).
Administration of the amino acid L-arginine stabilised this negative balance in two groups of animals (both LR and HR) in the series of experiments where L-arginine was introduced before and after the lead nitrate exposure, and these changes in the nitrite pool were statistically significant, compared with the overall effect of lead nitrate alone. We observed a statistically significant increase in nitrate levels in the group of HR animals receiving L-arginine, compared with the lead-nitrate group.
Thus, lead nitrate significantly reduced the levels of nitrites and nitrates, i.e. stable metabolites of the NO-related system, in both LR and HR animals, and the effect of the amino acid L-arginine can stabilise this negative lead nitrate effect in both groups showing a statistically significant increase in the levels of nitrites and nitrates. We also observed a statistically significant increase in nitrate levels in the HR group, compared to the lead nitrate group. This conclusion highlights the importance of assessing changes in the NO-related system in the context of lead-induced toxicity and the potential use of L-arginine as a stabilising agent for these changes, depending on the basal level of physiological reactivity of animals (LR and HR rats).
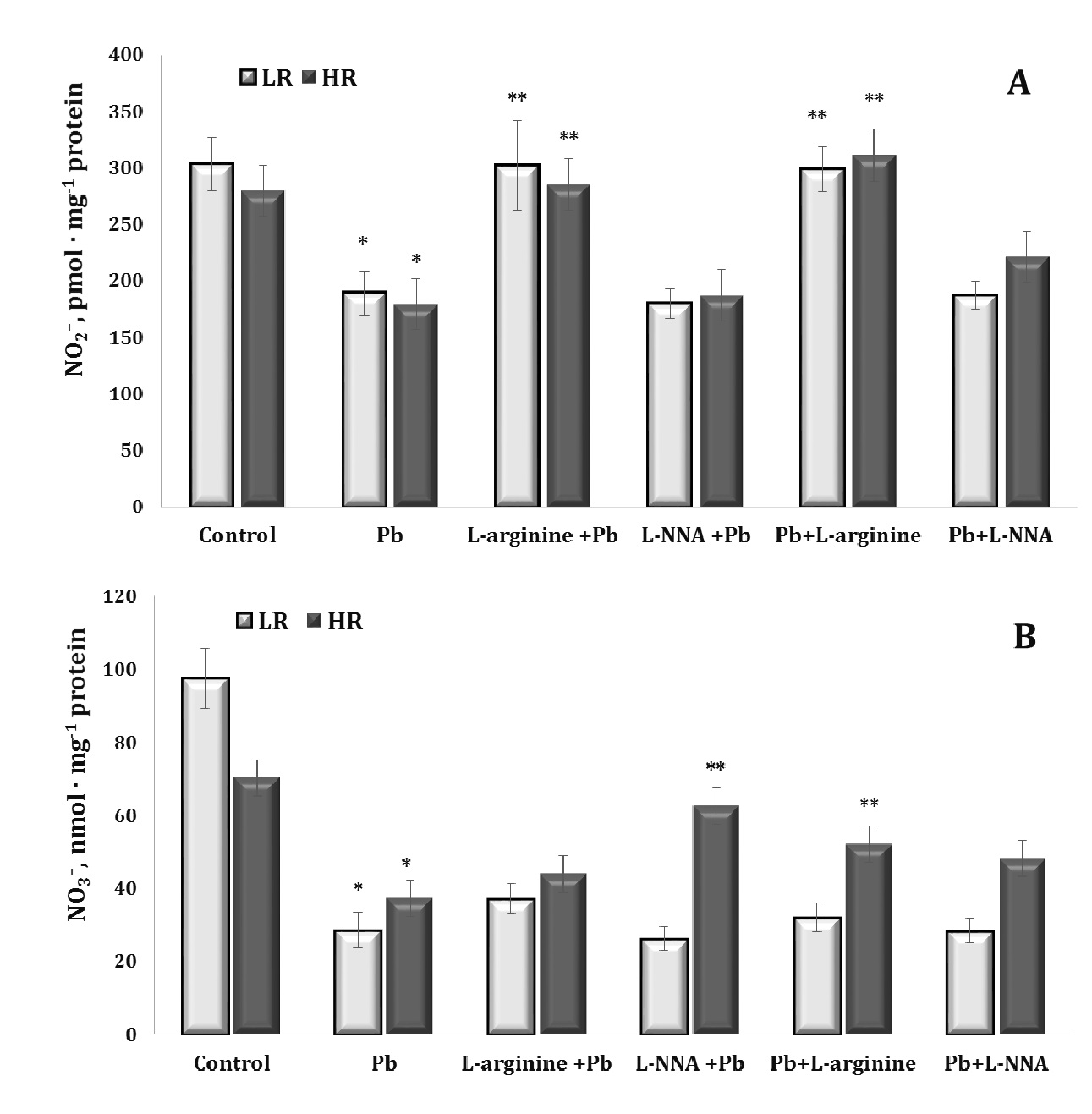
Fig. 3: Levels of nitrite (NO2–, A) and nitrate (NO3–, B) in the liver tissue of rats with low (LR) and high (HR) hypoxia resistance exposed to lead nitrate for 30 days and treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before and after exposure to lead nitrate. Data are expressed as mean ± S.E.M. (n = 6). * Significant differences between Pb group and untreated control (p<0.05); ** Significant differences between Pb and L-arginine, Pb and L-NNA treated groups (p<0.05).
Analysis of liver mitochondrial respiratory function
The next stage of our study was to assess mitochondrial respiratory function using various respiratory substrates. The results of this series of experiments are summarised in Tables 1-4. Our concept was based on the fact that prolonged lead-induced exposure (30 days in our experiment) can damage the function of mitochondria and Krebs cycle enzymes, such as dehydrogenases, affecting their ability to efficiently metabolise different substrates, including SC, KGL, and mixtures of oxidation substrates (3 mM glutamate + 3 mM pyruvate and 3 mM glutamate + 2.5 mM malate), leading to enhanced transamination processes. These dependencies can be explained by differences in the resistance of individual animals to hypoxia and the timing of administration of the amino acids L-arginine and L-NNA. These processes, in turn, can lead to impaired ATP production and significant changes in mitochondrial metabolism and function.
A statistically significant increase in the RCR ratio was observed in LR rats exposed to lead nitrate at SC oxidation, and these changes were significantly more pronounced than in the HR group of animals (Table 1). The administration of L-arginine prior to each administration of lead nitrate to the LR animals resulted in a statistically significant decrease in the rate of oxidative phosphorylation, RCR, but also a significant increase in the ADP/O ratio. These trends of increased mitochondrial respiratory efficiency at SC oxidation as an effect of the L-arginine administration prior to the lead nitrate exposure were more pronounced in the LR rats than in the HR rats. These features of the functioning of different metabolic pathways of mitochondrial energy supply (I and II sites of the mitochondrial respiratory chain as protein complexes in the electron transport chain responsible for the capture and transfer of electrons during oxidative phosphorylation in mitochondria) and the involvement of L-arginine in them as a nitric oxide precursor, are clarified by the results of our study on the effects of the NO synthase inhibitor L-NNA, precisely for in the HR group of rats, when L-NNA was administered both before and after the lead nitrate exposure. Namely, it this refers to the improvement of SC efficiency in liver mitochondria of the HR rats, defined by the increase of in state 3 mitochondrial respiration, RCR, and phosphorylation efficiency, compared to the LR rats.
The imbalance in the redistribution of oxidation substrates in the mitochondria at significant loads, one of which is lead poisoning, was verified by the parameters of mitochondrial oxygen consumption obtained at the oxidation of KGL as a substrate of the group of NADH-dependent substrates. The results are shown in Table 2. It should be noted that it is the ratio of SC-dependent to KGL-dependent oxidation in the mitochondria that is significantly different in the control in the two groups of animals studied in this study (LR and HR rats). In fact, the efficiency of KGL oxidation in individuals with high resistance to hypoxia was manifested in higher values of the rate of mitochondrial respiration in state 3, the RCR ratio, and the efficiency of oxygen consumption and oxidative phosphorylation for metabolic pathways in the mitochondria. However, it is precisely these processes that are most vulnerable to various stressors, such as lead poisoning in our study, and switch to predominantly intensive oxidation of SC with low RCR and efficiency of oxygen consumption and oxidative phosphorylation, which invariably leads to increased mitochondrial damage and increased levels of oxidative stress. Such peculiarities of mitochondrial processes were revealed in this study when we compared SC and KGL oxidation in mitochondria as a function of individual animal resistance.
The effects of L-arginine as a precursor of NO synthesis, both before and after the exposure to lead nitrate, are related to the effects of the increasing efficiency of oxygen consumption, especially in the LR group of rats, since they were completely abolished in similar conditions when the nitric oxide synthase inhibitor, L-NNA, was administered instead of L-arginine. It is important to note that the group of animals with HR exhibited an increase in the efficiency of mitochondrial respiratory processes when L-NNA and lead nitrate were administered. These processes were caused by the preservation of the main characteristics of the RCR and the ADP/O ratio at low values of the mitochondrial respiration rate in state 3, which was particularly manifested during the administration of L-NNA after the exposure to lead nitrate.
The next stage of our study was to use a mixture of mitochondrial respiratory substrates, i.e. glutamate, pyruvate, and malate, to assess the processes of ADP-stimulated mitochondrial oxidation during the lead nitrate exposure and the administration of L-arginine and L-NNA. This approach is justified because the combination of glutamate with pyruvate or malate is likely to provide additional substrates and cofactors necessary for the transamination reactions to proceed efficiently. This results in enhanced transamination processes, facilitating the synthesis of amino acids and the regeneration of key metabolites involved in cellular metabolism. The results of this series of experiments are summarised in Tables 3 and 4. We therefore investigated the preservation and maintenance of the NADH-dependent oxidation ‘pool’ during the lead nitrate exposure and the involvement of the NO-related system in these processes.
It is known that pyruvate is a key intermediate in glycolysis and acts as a keto acid in transamination reactions; when combined with glutamate, it is likely to act as an amino group acceptor leading to the formation of alanine via transamination. This process can regenerate glutamate, thereby increasing transamination overall. The next very important pathways involve malate, which can be converted to oxaloacetate, acting as a precursor for aspartate synthesis via transamination with glutamate. This process produces α-ketoglutarate, which can then be recycled to regenerate glutamate; this in turn promotes transamination reactions.
The results of our experiments showed a significant decrease in the rate of mitochondrial respiration in state 3 when using glutamate and pyruvate or glutamate and malate as substrates in mitochondrial oxidation, RCR, and the efficiency of the oxidative phosphorylation process itself in the liver mitochondria of rats exposed to lead nitrate in both groups of animals with LR and HR (Tables 3 and 4). The maintenance of the aminotransferase mechanism is therefore an essential link in the synthesis of amino acids and the regeneration of key metabolites involved in cellular metabolism under the influence of lead nitrate. The enhancement of the NO-dependent system by administration of the amino acid L-arginine restores this mitochondrial imbalance in LR rats, increasing the rate of mitochondrial respiration in state 3, RCR, and the efficiency of mitochondrial respiration and oxidative phosphorylation, which is important for maintaining their stabilising function in the generation of free radicals and the increase in lead-induced oxidative stress. This concept was confirmed in our experiments with the nitric oxide synthesis inhibitor L-NNA in the LR animals. It should be noted that the efficiency of using glutamate and malate as substrates for oxidation was higher in the effects of L-arginine in the HR rats than in the effects of L-NNA (Table 4).
Thus, the assessment of the efficiency of the functioning of the mitochondrial energy supply processes shows greater vulnerability of NADH-dependent oxidation at the exposure to lead nitrate used in the present study and is more pronounced in the organism of HR rats. The use of L-arginine in our experiments initiated the processes of oxidation of NADH-dependent substrates (KGL, first of all, in comparison with SC) in the group of the LR rats, but this directionality of the processes was more effective when the role of the NO system was reduced (use of L-NNA) in the HR animal group.
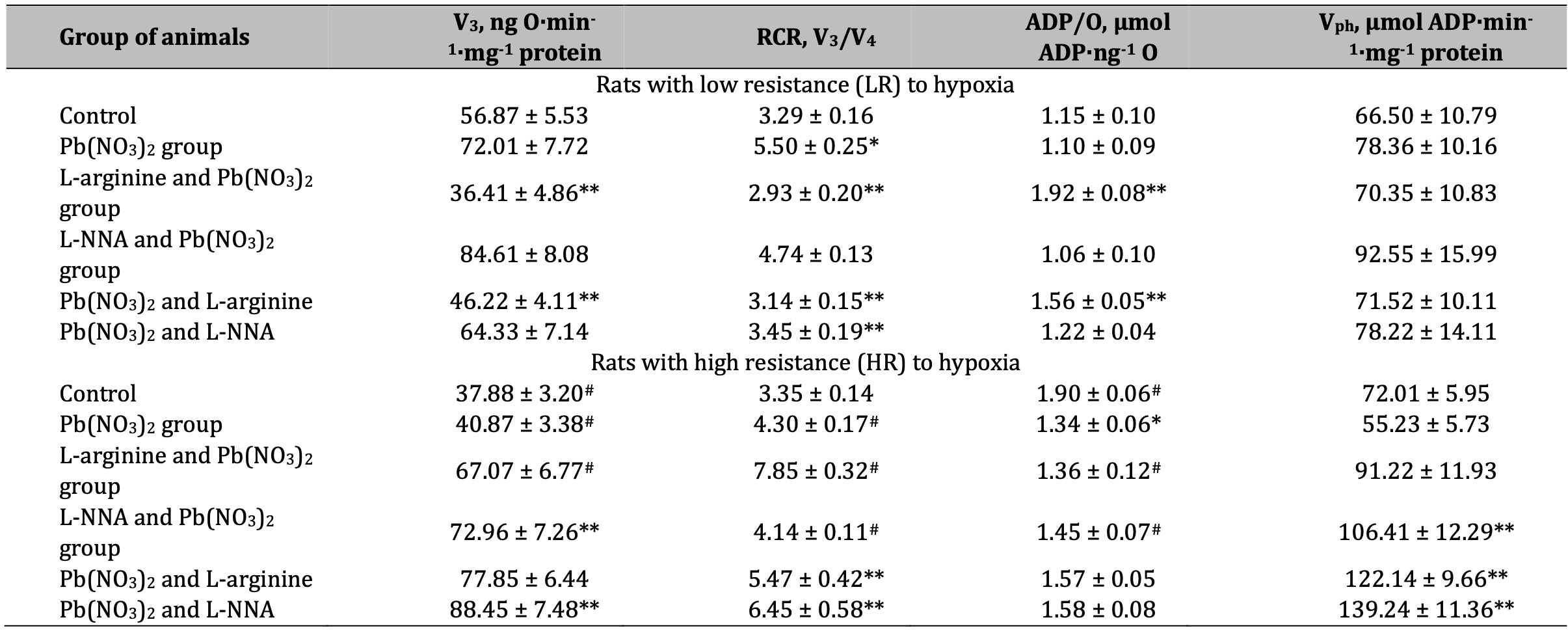
Table 1: Parameters of ADP-stimulated oxidative phosphorylation processes in the hepatic mitochondria of rats with low (LR) and high (HR) hypoxia resistance exposed to lead nitrate for 30 days and treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before and after exposure to lead nitrate. Oxidative substrate – 0.35 mM succinate. Data expressed as mean ± S.E.M. (n = 6). * Significant differences between Pb group and untreated control (p < 0.05); ** Significant differences between Pb and L-arginine, Pb and L-NNA treated groups (p < 0.05); # Significant differences between low and high resistance rats (p < 0.05)
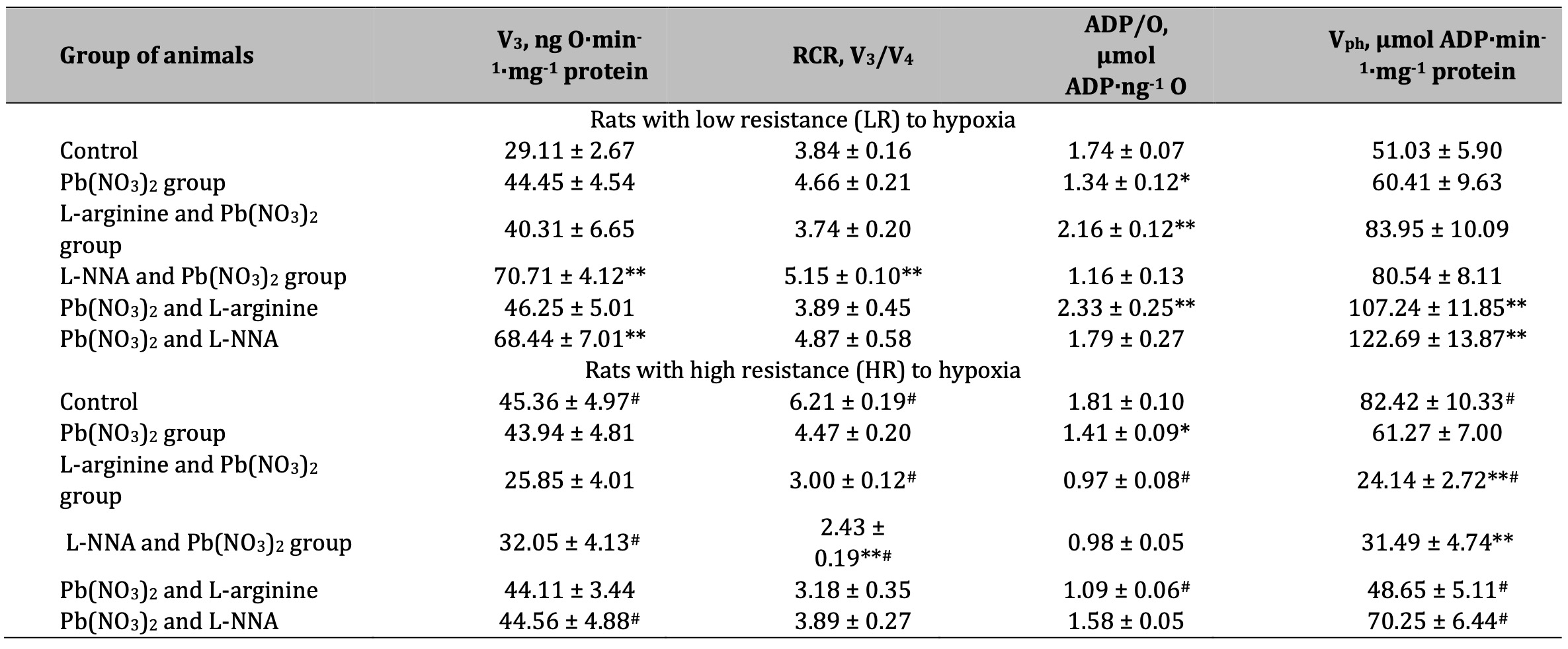
Table 2: Parameters of ADP-stimulated oxidative phosphorylation processes in the hepatic mitochondria of rats with low (LR) and high (HR) hypoxia resistance exposed to lead nitrate for 30 days and treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before and after exposure to lead nitrate. Oxidative substrates – 1 mM α-ketoglutarate. Data expressed as mean ± S.E.M. (n = 6). * Significant differences between Pb group and untreated control (p < 0.05); ** Significant differences between Pb and L-arginine, Pb and L-NNA treated groups (p < 0.05); # Significant differences between low and high resistance rats (p < 0.05)
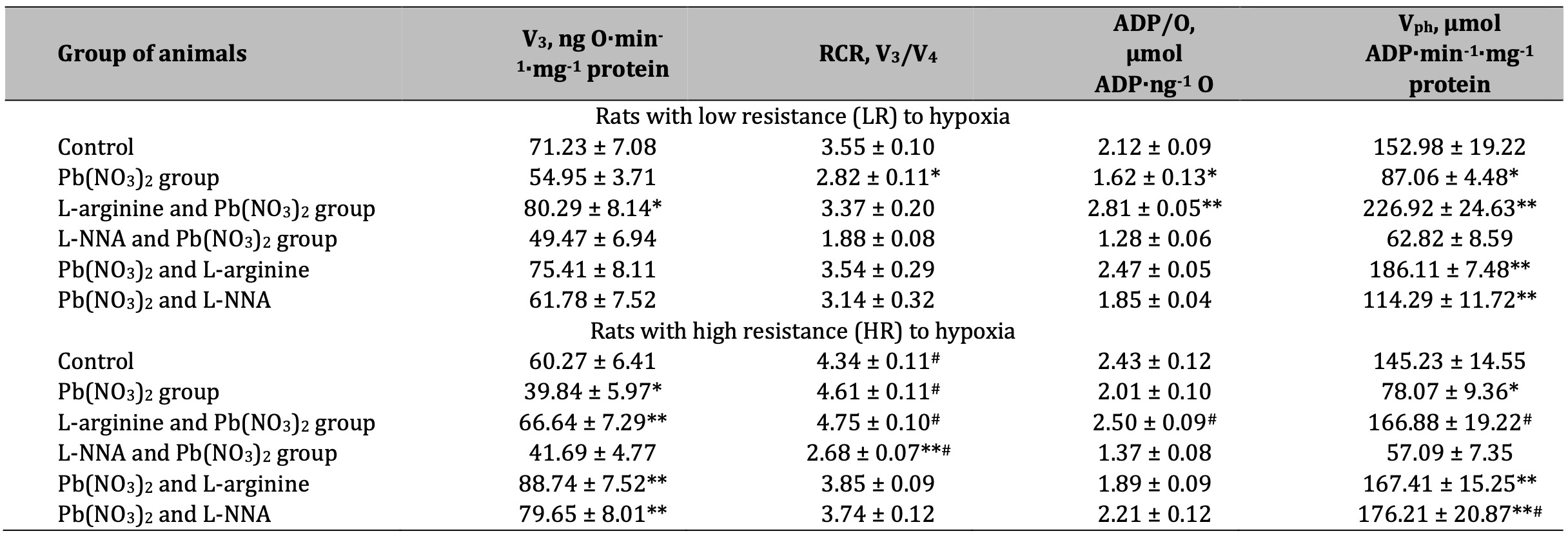
Table 3: Parameters of ADP-stimulated oxidative phosphorylation processes in the hepatic mitochondria of rats with low (LR) and high (HR) hypoxia resistance exposed to lead nitrate for 30 days and treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before and after exposure to lead nitrate. Oxidative substrates – 3 mM glutamate+3 mM pyruvate. Data expressed as mean ± S.E.M. (n = 6). * Significant differences between Pb group and untreated control (p < 0.05); ** Significant differences between Pb and L-arginine, Pb and L-NNA treated groups (p < 0.05); # Significant differences between low and high resistance rats (p < 0.05)
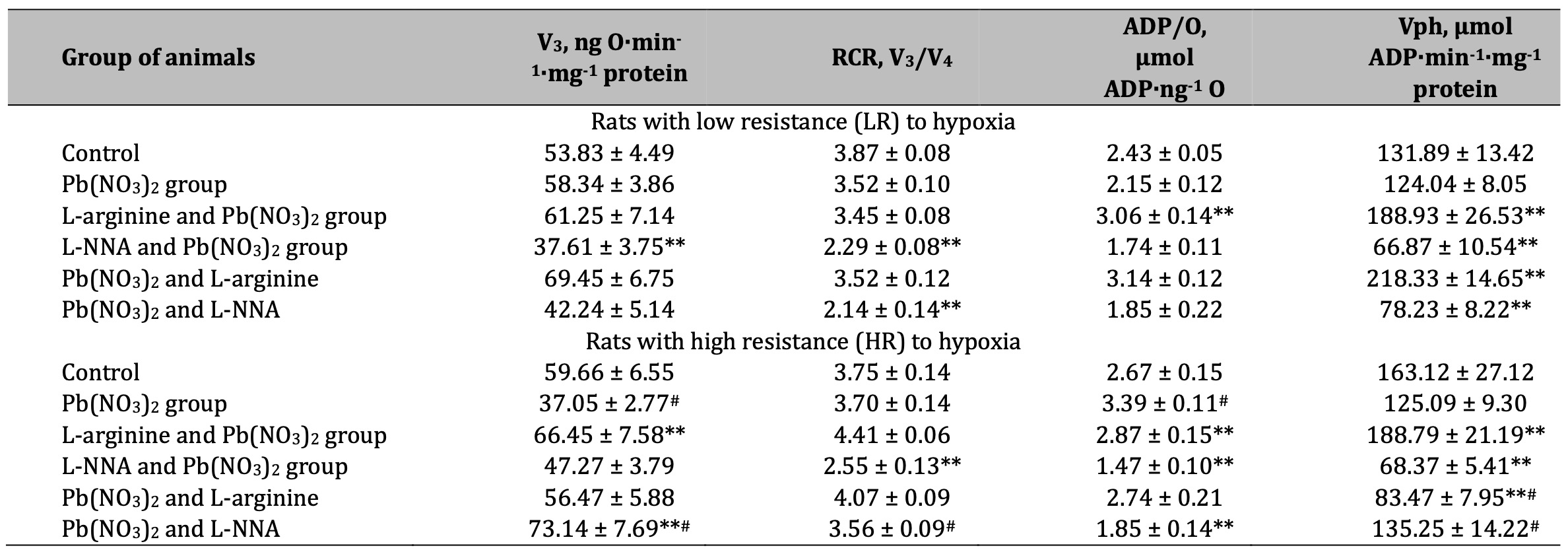
Table 4: Parameters of ADP-stimulated oxidative phosphorylation processes in the hepatic mitochondria of rats with low (LR) and high (HR) hypoxia resistance exposed to lead nitrate for 30 days and treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before and after exposure to lead nitrate. Oxidative substrates – 3 mM glutamate+2.5 mM malate. Data expressed as mean ± S.E.M. (n = 6). * Significant differences between Pb group and untreated control (p < 0.05); ** Significant differences between Pb and L-arginine, Pb and L-NNA treated groups (p < 0.05); # Significant differences between low and high resistance rats (p < 0.05)
Aminopyrine N-demethylase activity as a biomarker of microsomal oxidation function
Cytochrome P450 is known to play a crucial role in the metabolism of various compounds, including toxins, drugs, and chemicals; in the context of lead poisoning, assessment of microsomal oxidation biomarkers may reveal how the body processes lead. The results of this series of experiments are shown in Fig. 4.
The effect of lead nitrate caused a statistically significant twofold increase in the level of aminopyrine N-demethylase activity in the liver, which was significantly reduced in the experiments with the L-arginine administration to the rats. This redistribution of the level of oxygen-dependent processes towards mitochondrial oxygen-dependent processes under the influence of the nitric oxide precursor amino acid L-arginine is an important mechanism for maintaining the function of the mitochondrial respiratory chain, since the activity of dehydrogenases is significantly modified by lead nitrate administration.
No less importantly, we obtained statistically significant differences between the groups of HR and LR animals in the control group and those exposed to lead nitrate. It should be noted that the levels of the biomarker of microsomal oxidation were significantly higher in the HR groups in our experiment than in the LR individuals. This one of the characteristic features in the functioning of oxygen-dependent processes in the organism of HR animals (mitochondrial respiration, microsomal oxidation, intensity of lipoperoxidation processes) may give the advantages of effective redistribution of metabolic pathways with participation of oxygen-dependent processes.
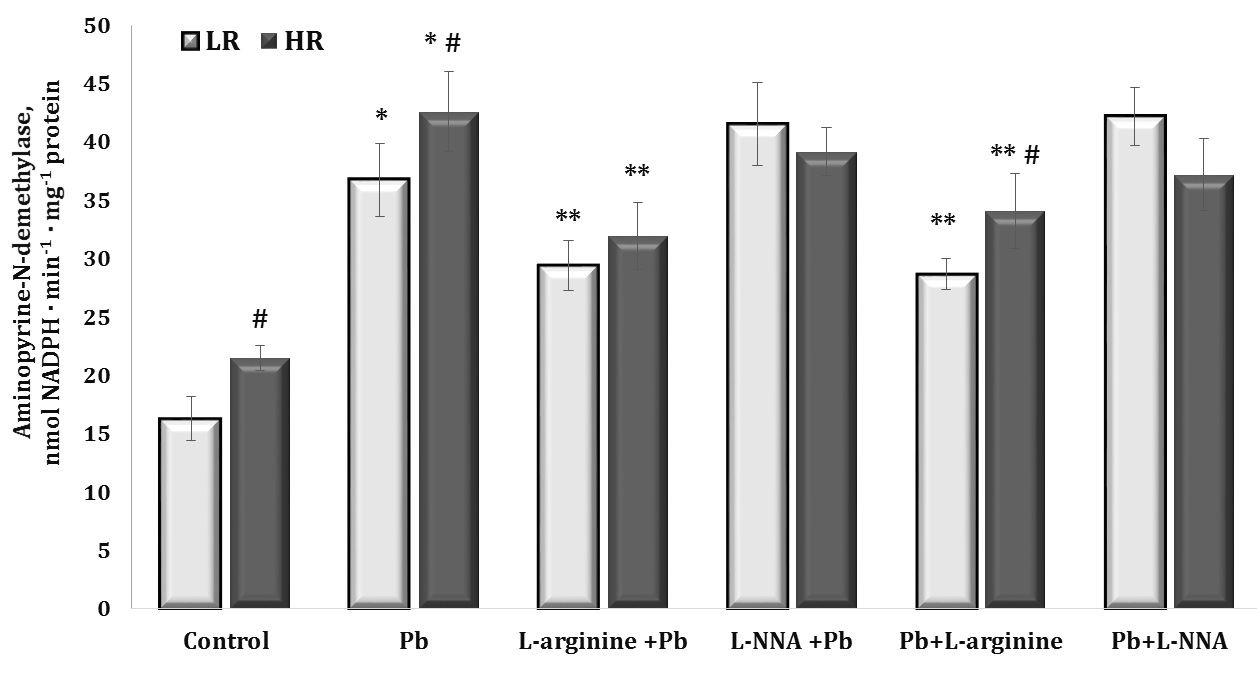
Fig. 4: Aminopyrine-N-demethylase activity (nmol NADPH · min-1 · mg-1 protein) in the liver tissue of rats with low (LR) and high (HR) hypoxia resistance exposed to lead nitrate for 30 days and treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before and after exposure to lead nitrate. Data are expressed as mean ± S.E.M. (n = 6). * Significant differences between Pb group and untreated control (p<0.05); ** Significant differences between Pb and L-arginine, Pb and L-NNA treated groups (p<0.05); # Significant differences between LR and HR (p<0.05).
Oxidative stress and antioxidant defences
The next part of our study was to assess the impact of lead nitrate administration on the levels of diene conjugation as the first initiating stage and TBARS as a biomarker of the final stage of lipid peroxidation processes and its potential implications for organisms and the environment. Investigations of the interaction of lead nitrate may provide a better understanding of the toxic mechanisms of this metal and its role in the formation of harmful chemical reaction products. These data are presented in Fig. 5 (A and B).
The effects of the lead nitrate exposure caused a significant more than twofold increase in the levels of diene conjugates in liver tissue, and these changes were more pronounced in the HR animals than in the LR animals. The exogenous L-arginine treatment contributed to the reduction of diene conjugate levels in both groups of the LR and HR rats after lead nitrate intoxication, but these effects were more pronounced in the LR animals. The effects of L-NNA were associated with the abolition of the effects of L-arginine only in the group of animals with LR. In turn, and a statistically significant decrease in the levels of diene conjugates was observed in the HR rats, compared with the group of rats given lead nitrate alone. The administration of the nitric oxide precursor and inhibitor of NO biosynthesis after the lead nitrate exposure to the two groups of rats showed similar tendencies of directionality of effects as described above.
The study of the content of lipid peroxidation end products by the level of TBARS in liver tissue showed a pronounced protective effect of the amino acid L-arginine or nitric oxide in lead nitrate poisoning, but these dependencies had a clearly marked pattern related to individual physiological reactivity. These dependencies consisted in a decrease in the level of the final products of lipid peroxidation in the animals with LR during lead nitrate poisoning observed over 30 days when L-arginine was administered to the rats with LR, and when a nitric oxide synthase inhibitor, L-NNA, was administered in the rats with HR.
As the assessment of aldehydic and ketonic derivatives of protein oxidative modifications is crucial for understanding the mechanisms of lead toxicity and for the development of potential therapies or preventive strategies in cases of lead poisoning, we focused on this part of our study, as shown in Fig. 5 (C and D). As shown in these figures, upon the exposure to lead nitrate, such functional groups as amino and thiol groups may have undergone oxidative modification as a result of interactions of lead with proteins, leading to increased formation of aldehydic (OMP AD) and ketonic (OMP KD) derivatives. These modified proteins may have lost their functionality, leading to disturbances in cell and tissue function. Exogenous L-arginine prevented these lead-induced changes by reducing the levels of aldehyde and ketone derivatives of proteins, whereas L-NNA significantly activated these processes, especially in the LR group of animals.
Thus, our study highlighted the pronounced protective effect of L-arginine in lead nitrate poisoning, as evidenced by the significant reduction in the levels of lipid peroxidation end products in liver tissue. However, these effects were influenced by individual physiological reactivity. Specifically, the animals with low reactivity showed reduced levels of lipid peroxidation products when given L-arginine, whereas the individuals with high reactivity showed reduced levels when given the nitric oxide synthase inhibitor, L-NNA.
In the context of lead nitrate exposure, the co-operation of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx), is crucial to neutralise ROS and prevent oxidative damage. However, under lead nitrate exposure, excessive ROS generation can exceed the body’s antioxidant capacity to neutralise these ROS, leading to excessive oxidative stress and cell damage. These relationships are illustrated in Fig. 6.
As we have previously shown in relation to the levels of diene conjugates and TBARS as biomarkers of lipid peroxidation, with lead nitrate exposure, the excess production of ROS exceeds the antioxidant capacity of the body to neutralise these ROS, resulting in excessive oxidative stress and cellular damage. As shown in our study, lead nitrate affects the activity of antioxidant enzymes: SOD (Fig. 6A), CAT (Fig. 6B), GR (Fig. 6C), and GPx (Fig. 6D) by reducing their activity. This in turn impairs the body’s ability to neutralise ROS and increases susceptibility to oxidative damage caused by long-term lead exposure.
In our study of the activity of key antioxidant enzymes in rat liver tissue during lead nitrate exposure, changes in the catalase-peroxidase ratio were observed. We showed different activity trends for SOD, CAT, GPx, and GR, which responded differently to the lead nitrate toxicity. We showed a statistically significant decrease in the activity of SOD, i.e. the enzyme responsible for scavenging the superoxide anion radical, which can lead to the formation of other more active forms of oxygen. Exposure to lead nitrate has been shown to decrease SOD activity, which impairs the body’s ability to neutralise these radicals, leading to increased oxidative stress.
In contrast to the reduced SOD activity, we have shown a significant increase in CAT activity, possibly in response to the increased levels of hydrogen peroxide (H2O2), which is a substrate for this enzyme. Under lead nitrate exposure, the body may increase catalase production in an attempt to neutralise this toxic substrate. Increased catalase activity is an attempt by the body to reduce excess H2O2, which is toxic to cells, and this tendency did not change in our experiments when both the nitric oxide donor and the nitric oxide biosynthesis inhibitor were administered. We also showed a significant decrease in the activity of GPx, which is involved in reactions with lipid peroxides formed as a result of lipid peroxidation. GPx is a glutathione-dependent enzyme and a key enzyme in neutralising hydrogen peroxide by reacting with reduced glutathione (GSH) to form water and oxidised glutathione. This reaction helps to remove peroxides, which can be toxic to cells and contribute to damage to DNA, proteins, and lipids.
We have also shown a decrease in the activity of GR, an enzyme that regenerates GSH from its oxidised form (GSSG) (Lu, 2009, 2013). These important mechanisms of lead toxicity may result in reduced availability of glutathione, a substrate for GPx, or may directly affect the structure of GPx by reducing the activity of this enzyme (Devóz et al., 2021). Thus, in our study, the exposure to lead nitrate significantly impaired the function of both glutathione metabolising enzymes, resulting in reduced availability of reduced glutathione, an important antioxidant in cells. Thus, as a result of these changes in peroxidase and catalase activities induced by the lead nitrate exposure, there was a significant increase in oxidative stress and a marked decrease in antioxidant defences and total antioxidant capacity in liver tissue (Fig. 7), which inevitably leads to cell and tissue damage and metabolic disorders, with a number of negative consequences for the organism.
The changes in the activity of key antioxidant defence enzymes in the rat liver tissue were pronounced and statistically dependent in the groups according to the individual baseline physiological reactivity in our study of the effects of the lead nitrate exposure. For all the four antioxidant enzymes studied, the changes induced by L-arginine upon the exposure to lead nitrate were of a normalising nature. When we examined the effect of the nitric oxide synthase inhibitor L-NNA in the group of the highly resistant rats after the lead nitrate exposure, we observed an increase in the activity of SOD, GR, and GPx and a decrease in CAT activity. We also observed an increase in GPx activity in the HR rats when L-NNA was administered prior to the lead nitrate exposure. We demonstrated a statistically significant increase in GPx activity in the LR group when L-arginine was administered to the animals both before and after the exposure to lead nitrate. Thus, these data highlight the complex interplay between lead nitrate toxicity and antioxidant enzyme activity and suggest potential avenues for therapeutic intervention or modulation of baseline physiological responses to mitigate the adverse effects of lead nitrate exposure.
Our study also focused on assessing the impact of the lead nitrate exposure on total antioxidant capacity (TAC) as a measure of the organism’s ability to counteract oxidative stress by neutralising free radicals. A higher TAC value indicates a greater amount of antioxidants present in the biosystem and a better ability to protect against oxidative stress. These data are shown in Fig. 7.
As can be seen from these data, the exposure to lead nitrate resulted in a significant increase in oxidative stress in the body by increasing the production of free radicals. The activation of antioxidants to neutralise these toxic substances was significantly reduced, and the function of antioxidant enzymes was predominantly reduced, especially in the LR animals. The administration of L-arginine contributed to a significant increase in antioxidant defences against the lead nitrate exposure in both groups of animals (HR and LR). L-arginine is a precursor for the biosynthesis of NO, which acts as an antioxidant and protects cells from oxidative stress. Therefore, administration of L-arginine may enhance NO synthesis, which in turn may contribute to an increase in TAC (Fig. 7). L-arginine contributed to the increase in TAC both before and after the exposure to lead nitrate. However, we also obtained statistically significant effects, although significantly lower than the effects of L-arginine, on the increase in TAC levels during the administration of L-NNA. Thus, an increase in TAC levels following L-arginine administration in cases of lead nitrate exposure may indicate the ability of L-arginine to stimulate the production of antioxidants in the body, which may be beneficial in reducing oxidative stress and its negative effects. Nitric oxide produced by L-arginine plays an important role as an antioxidant, supporting the body’s defence against oxidative stress.
Notably, the effects of L-arginine on the increasing TAC levels were evident both before and after the exposure to lead nitrate. In addition, our results showed statistically significant albeit significantly smaller effects than that of L-arginine, with administration of the L-NNA synthase inhibitor also increasing the TAC levels. These findings highlight the complex interplay between lead nitrate toxicity, antioxidant defences, and the potential therapeutic treatment with L-arginine.
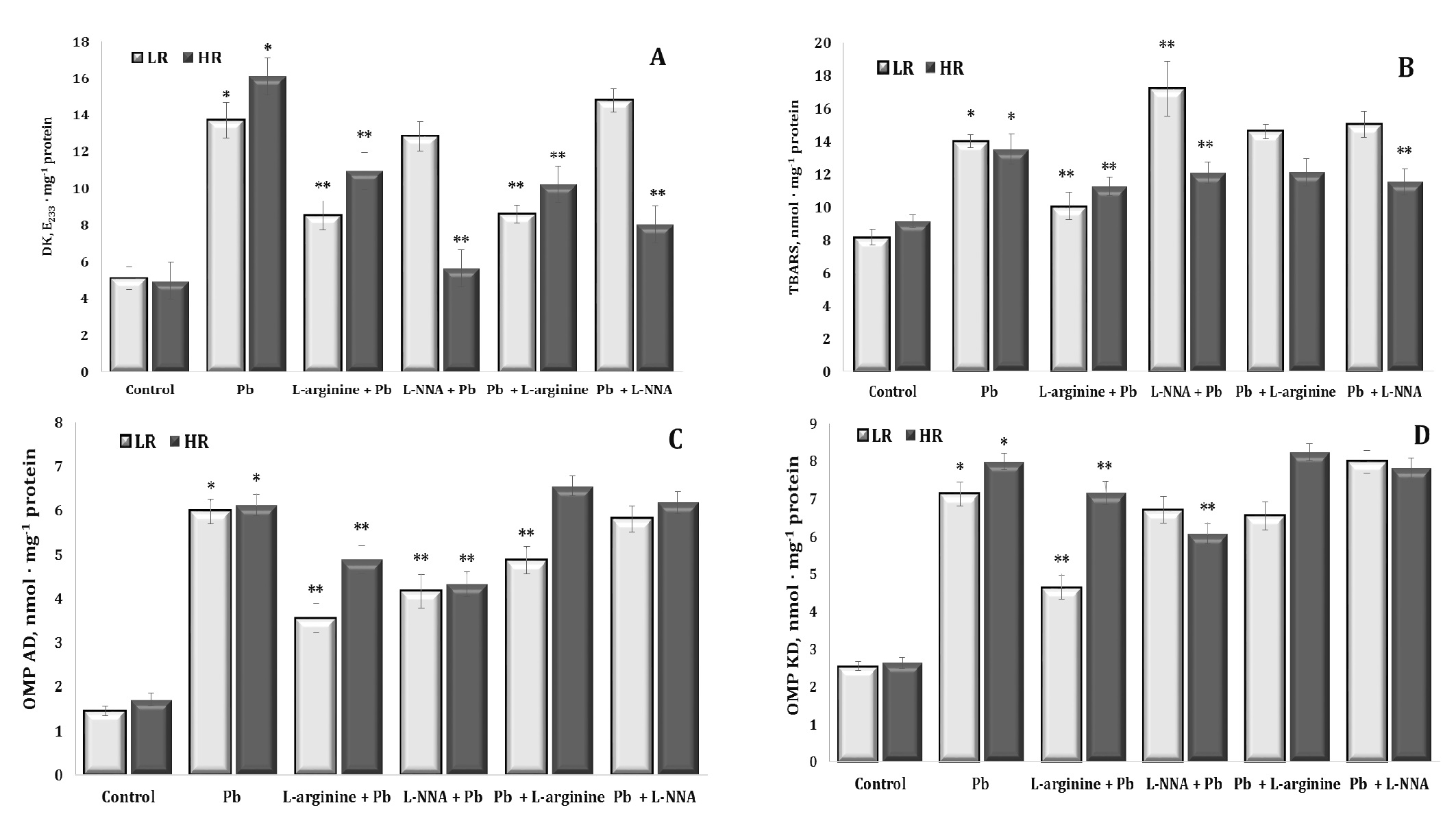
Fig. 5: Levels of diene conjugates (A, DK, E233 · mg-1 protein), 2-thiobarbituric acid reactive substances (B, TBARS, nmol · mg-1 protein), aldehydic derivatives of oxidatively modified proteins (C, OMP AD, nmol · mg-1 protein) and ketonic derivatives of oxidatively modified proteins (D, OMP KD, nmol · mg-1 protein) in the liver tissue of rats with low (LR) and high (HR) hypoxia resistance exposed to lead nitrate for 30 days and treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before and after exposure to lead nitrate. Data are expressed as mean ± S.E.M. (n = 6). * Significant differences between Pb group and untreated control (p<0.05); ** Significant differences between Pb and L-arginine, Pb and L-NNA treated groups (p<0.05).
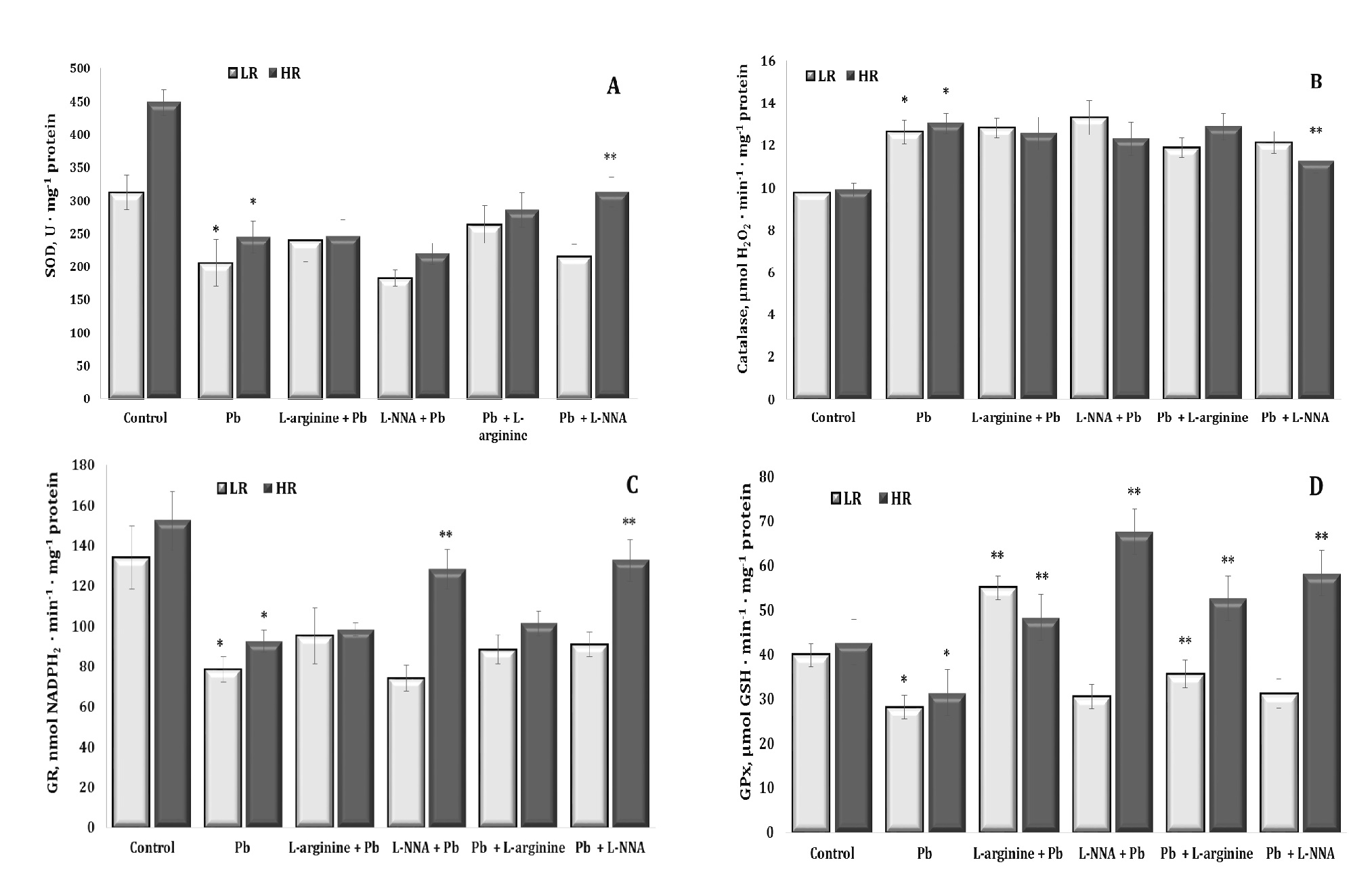
Fig. 6: Activity of superoxide dismutase (A, SOD, U ∙ mg-1 protein, A), catalase (B, μmol H2O2 · min-1 · mg-1 protein), glutathione reductase (C, GR, nmol NADPH2 · min-1 · mg-1 protein), and glutathione peroxidase (D, GPx, nmol GSH · min-1 · mg-1 protein) in the liver tissue of rats with low (LR) and high (HR) hypoxia resistance exposed to lead nitrate for 30 days and treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before and after exposure to lead nitrate. Data are expressed as mean ± S.E.M. (n = 6). * Significant differences between Pb group and untreated control (p<0.05); ** Significant differences between Pb and L-arginine, Pb and L-NNA treated groups (p<0.05).
Discussion
The aim of this study was to investigate the interplay between mitochondrial oxygen consumption, microsomal oxidation, oxidative stress-inducing processes, and antioxidant defences in the context of individual resistance to hypoxia under lead nitrate exposure and treatment with L-arginine as a precursor of NO synthesis and L-NNA as an inhibitor of NO synthase, administered for 30 days before and after lead nitrate exposure. The main aspects of our study are outlined below.
First, through comprehensive biochemical analyses, we have gained valuable insights into variations in mitochondrial oxygen consumption, levels of oxidative stress biomarkers and antioxidant defence enzymes, and total antioxidant capacity as a function of the individual physiological reactivity of the animals. Our study of the relationship between the effects of L-arginine, which is associated with nitric oxide synthesis, and the inhibition of these mechanisms by the NO synthase inhibitor L-NNA, administered before and after the exposure to lead nitrate, helped to analyse whether the level of nitric oxide production influenced the ability to neutralise the toxic effects of the lead nitrate exposure. The differences in the effects of L-arginine and L-NNA administration may be related to the regulation of nitric oxide levels in the body. Administration of L-arginine may increase its levels, which may be beneficial in the context of lead nitrate exposure due to the potential protective effects of NO. In turn, L-NNA administration may reduce NO production, which may limit these potential benefits.
In this study, the results showed that the exposure to lead nitrate significantly increased the RCR in liver mitochondria from rats with LR and decreased the ADP/O ratio in liver mitochondria from rats with HR to succinate oxidation (Table 1). FADH-dependent oxidation was the most resistant to the lead nitrate-induced damage. L-arginine reduced the rate in state 3 and RCR and restored the ADP/O ratio in the succinate oxidation conditions only in the LR rats (Table 1). L-arginine had a greater effect on mitochondrial oxygen consumption in the LR rats than in the HR rats. The exposure to L-arginine and lead nitrate increased the ADP/O ratio with both NADH- and FADH-dependent substrates and via the transamination pathway. L-arginine protected the liver of the LR rats from the cytotoxic effects of lead nitrate, as assessed by the mitochondrial oxygen consumption assay (Table 1). These changes are consistent with the overall improvement in the mitochondrial function in the liver of the LR rats: improvement in the rate of oxidative phosphorylation, in the coupling of oxidative phosphorylation (increased RCR), and in the mitochondrial efficiency of oxidative phosphorylation (ADP/O ratio) when KGL was used as a substrate and when both glutamate and pyruvate and glutamate and malate were used via the transamination pathway (Tables 1-4). The RCR in the lead nitrate-treated HR rats under the NADH-dependent substrate oxidation in mitochondria was significantly decreased by the L-NNA administration (Table 2). The RCR in the lead nitrate-treated HR rats administered L-NNA were significantly decreased under the NADH-dependent substrate oxidation in liver mitochondria (Table 2).
These studies are important due to the role of NO in preventing lipid peroxidation, which is a key event in the pathology of several diseases, including liver disease. In this study, we attempted to elucidate the role of NO in lead nitrate exposure. Nitric oxide is known to act as both a promoter and an inhibitor of lipid peroxidation, making its role in oxidative processes complex and context dependent (Star, 1993; Cyr et al., 2020). By itself, NO acts as a potent inhibitor of the lipid peroxidation chain reaction by scavenging propagating lipid peroxyl radicals (Lundberg and Weitzberg, 2020). It can also inhibit many potential lipid peroxidation initiators via enzymes (Oral, 2021). However, in the presence of superoxide anions, NO forms peroxynitrite, i.e. a potent oxidant that can initiate lipid peroxidation and oxidise lipid-soluble antioxidants (Kiani et al., 2022; Fujii and Yamada, 2023). Whether NO protects or induces injury is likely to be determined by the type of injury, the abundance of ROS, the source and amount of NO production, and the cellular redox status of the liver (Dios-Barbeito et al, 2022). Understanding the mechanisms that regulate the effect of NO on lipid peroxidation processes is crucial for elucidation of its impact on liver function and development of pathologies associated with lead poisoning (Shaban et al., 2022).
However, it is important to note that the effects of administration of these substances may depend on many factors, such as the dose, time of administration, and individual physiological characteristics of the organism. In mammals, NO is generated enzymatically by three isoforms of NO synthase (NOS), namely inducible NOS (iNOS), endothelial NOS (eNOS), and neuronal NOS (nNOS) (Poeggeler et al., 2023). The reactivity of NO depends on its physical properties, such as its small size, high diffusion rate, and lipophilicity (leading to its accumulation in hydrophobic regions) as well as its simple but selective chemical reactivity towards a variety of cellular targets (Wu et al., 2023). Therefore, experimental studies of these compounds in the context of lead poisoning in rats may help to better understand their role and influence on the course of this toxicity. It is known that higher levels of cholinergic NO-dependent mechanisms, as we have previously shown (Kurhaluk, 2023a,b), may be associated with a greater ability to protect against the toxic effects of lead nitrate through various mechanisms, such as neutralisation of ROS. These studies have demonstrated the detoxifying role of L-arginine in lead nitrate exposure through the activation of metabolic antioxidant mechanisms by preventing the activation of both initial (diene conjugation) and final (TBARS) products of lipid peroxidation.
The conclusion drawn from our study is that lead nitrate exposure may disrupt the functioning of the nitric oxide system, resulting in reduced reactivity and interaction with other regulatory factors. The assessment of the concentration of stable metabolites of its metabolism, such as nitrites and nitrates, proves to be a crucial element in the evaluation of metabolic changes related to lead exposure (Haswell-Elkins et al., 1994; Carmignani et al., 2000). Our experiments showed that lead nitrate significantly reduced the levels of nitrites and nitrates, i.e. the stable metabolic components of the nitric oxide system, in both the LR and HR groups of animals. However, the administration of L-arginine stabilised this negative effect in both groups, showing a statistically significant effect. We also observed a statistically significant increase in nitrate levels in the HR group compared with the control group. This conclusion highlights the importance of assessing changes in the nitric oxide system in relation to lead exposure and the potential use of L-arginine as a treatment and preventive agent for these changes.
Secondly, the statistical analysis allowed us to identify correlations between the parameters of mitochondrial oxygen consumption, oxidative stress biomarkers, and antioxidant enzyme activities. It is known that the efficiency of NO storage is genetically determined and appears to be related to the inherited capacity for NO synthesis (Kurhaliuk, 2001). Individual resistance to hypoxia provides an individual response of mitochondrial respiratory chain functioning (Lukyanova et al., 2007), mitochondrial ion transport (Mironova et al., 2010), properties of mitochondrial enzymes and energy metabolism (Lukyanova et al., 2007; Kurhaluk, 2023a,b), activity of monooxygenase system as a system of biotransformation of xenobiotics, and the drug metabolism system (Bayanov and Brunt, 1999). The differences in the adenylate pool parameters in the hepatocytes from rats with low and high resistance to hypoxia indicate that energy metabolism is a mechanism involved in the formation of individual cell resistance to oxygen deprivation. Individual constitutional resistance to hypoxia can be a serious criterion for an individual approach to pharmacotherapy of hypoxic states and diseases and for the prognosis and prevention of early and distant complications of irrational pharmacotherapy (Lukyanova and Dudchenko, 2003).
In this study, we analysed the relationships between lead nitrate exposure and oxidative stress parameters in rats with different levels of resistance to hypoxia. Our aim was to understand the effects of lead nitrate on oxidative stress biomarkers and antioxidant defences in liver tissue and their differences according to the physiological reactivity of the animals. These correlations suggest significant relationships between lead nitrate exposure and oxidative stress biomarkers in rats with different levels of resistance to hypoxia. In the low-resistance group of rats, positive correlations were observed between the lead levels and the TBARS levels (r = 0.54, p = 0.000), CAT activity (r = 0.82, p = 0.000), and the OMP-AD levels (r = 0.51, p = 0.000), suggesting that the biomarkers of oxidative stress increased with the increasing lead levels. Conversely, in the highly resistant rat group, positive correlations were found between the lead levels and OMP-KD (r = 0.64, p = 0.000) and TAC (r = 0.72, p = 0.000), suggesting a similar trend of lead-induced oxidative stress, albeit with different parameters. These findings highlight the importance of individual resistance to hypoxia in modulating the effects of lead nitrate exposure on oxidative stress and antioxidant defence responses.
In this study, we assessed the effect of lead nitrate on TAC, which serves as a crucial measure of an organism’s ability to combat oxidative stress by neutralising free radicals. The higher the TAC, the greater the presence of antioxidants in the biosystem, indicating a superior ability to defend against oxidative stress. This correlation is visually shown in Fig. 7. Our results showed that the exposure to lead nitrate resulted in a significant escalation of oxidative stress in the body, primarily through increased production of free radicals. Interestingly, however, this increase in the biomarkers of oxidative stress was not accompanied by a corresponding increase in the production of antioxidants to neutralise these harmful substances. In addition, the functionality of antioxidant enzymes was significantly impaired, particularly in the LR group of animals.
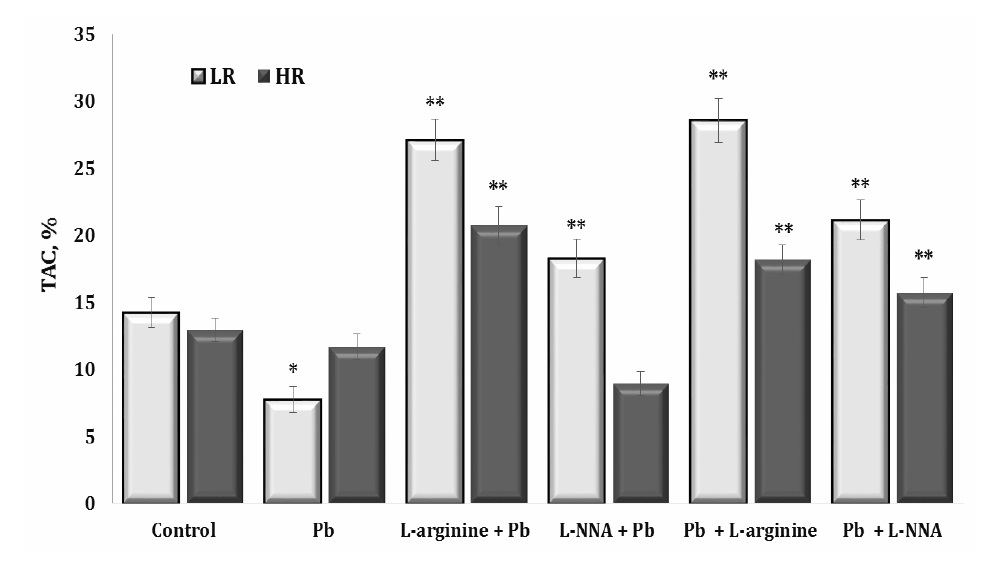
Fig. 7: The total antioxidant capacity (TAC) in the liver tissue of rats with low (LR) and high (HR) hypoxia resistance exposed to lead nitrate for 30 days and treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before and after exposure to lead nitrate. Data are expressed as mean ± S.E.M. (n = 6). * Significant differences between Pb group and untreated control (p<0.05); ** Significant differences between Pb and L-arginine, Pb and L-NNA treated groups (p<0.05).
The administration of L-arginine made a significant contribution to improving antioxidant defences under the lead nitrate exposure in both the HR and LR groups of animals. L-arginine serves as a precursor for the biosynthesis of NO, which acts as an antioxidant to protect cells from oxidative stress (Zhang et al., 2019). L-arginine therefore facilitates increased NO synthesis. This may have contributed to the increase in the TAC levels in our study.
Thirdly, our findings provide a deeper understanding of the physiological responses to lead nitrate exposure by shedding light on the molecular mechanisms underlying nitric oxide synthesis by L-arginine administration and its inhibition by L-NNA action. Our results highlight the importance of measuring the aldehydic and ketonic derivatives of oxidatively modified proteins in elucidation of the mechanisms of lead nitrate toxicity and in development of potential therapeutic interventions. Fig. 5 (C and D) shows that the exposure to lead nitrate led to oxidative modification of proteins, resulting in the formation of aldehydic and ketonic derivatives. These modifications affected the functionality of proteins and disrupted the function of cells and tissues. Exogenous L-arginine attenuated the effects induced by the exposure to lead nitrate by reducing the levels of aldehydic and ketonic derivatives of proteins. Conversely, L-NNA exacerbated these processes, especially in the LR animals. This highlights the need for tailored interventions based on individual physiological responses to effectively mitigate the toxic effects of lead nitrate.
We used the NO2– and NO3– concentration assay to investigate the effect of L-arginine and L-NNA on lead nitrate-induced oxidative stress. In this study, the lead nitrate exposure reduced liver NO2– and NO3–, i.e. markers of NO metabolism (Fig. 3). In addition, the lead nitrate exposure significantly reduced liver NO3– in the rats with LR, but the reduction in the nitrate levels in the HR rats was lower (by 47%, p = 0.029) than in the untreated controls. In both the LR and HR rats, L-arginine did not significantly increase the NO2– and NO3– levels. L-NNA, an inhibitor of NO synthase, significantly protected against lead-induced liver injury by increasing liver NO3– levels only in the HR rats. When NO3– levels are increased, either there is a systemic increase in the expression of NO synthase, which increases NO synthase coupling, or non-enzymatic sources lead to increased release of NO in the liver (Masri et al., 2005). Therefore, the ability of L-NNA to prevent lead-induced liver injury, as indicated by markers of NO metabolism, is limited to HR rats.
The influence of L-arginine on the increase in TAC was evident both before and after the exposure to lead nitrate. Furthermore, our results showed statistically significant therapeutic effects of L-arginine, albeit of a lesser magnitude, compared to the preventive effects of L-arginine. Interestingly, the administration of L-NNA also resulted in an increase in TAC levels. These results highlight the complex relationship between lead nitrate toxicity, antioxidant defences, and the potential therapeutic implications of interventions, such as L-arginine or L-NNA treatment. Identifying strategies to ameliorate the detrimental effects of lead nitrate exposure on oxidative stress and general health is a promising area for further research.
Fourthly, our research on the metabolism of L-arginine or L-NNA has considered the role of different dependencies based on individual physiological reactivity. Interactions between mitochondrial respiration, microsomal oxidation, and lipid peroxidation processes may play a key role in understanding the mechanisms of lead nitrate toxicity and the potential protective effects of L-arginine or L-NNA. In our research focusing on the influence of lead nitrate on the activity of key enzymes involved in antioxidant defence in rat liver tissue, significant changes were observed that were particularly pronounced and statistically correlated within the groups based on individual baseline physiological reactivity. Specifically, for all the four antioxidant enzymes studied, the changes induced by the lead nitrate exposure showed a tendency towards their normalisation during the L-arginine treatment.
In physiological conditions, the mitochondrial electron transport chain consumes more than 85% of the oxygen used by cells. It is estimated that approximately 1-5% of the oxygen consumed by mitochondria is converted to ROS, such as O2·– and H2O2 (Förstermann et al., 2017). Accumulating evidence has shown that mitochondria can produce and consume NO, which inhibits mitochondrial respiration via acute and reversible inhibition of cytochrome oxidase by NO in competition with O2 and irreversible inhibition at multiple sites by reactive nitrogen species (Galkin et al., 2007). Higher concentrations of NO and its derivatives (peroxynitrite, nitrogen dioxide, or nitrosothiols) can lead to irreversible inhibition of the respiratory chain, uncoupling, permeability transition, and/or cell death (Stewart et al., 2000; Radi et al., 2002). NO and reactive nitrogen species inhibit respiratory complexes I, III, and IV, leading to the production of superoxide, which reacts with NO to form peroxynitrite (ONOO–). This further inhibits the respiratory chain, aconitase, and Mn-superoxide dismutase (Mn-SOD) (Brown and Borutaite, 2007).
In addition, our study of the effects of the nitric oxide synthase inhibitor L-NNA in a group of HR rats following exposure to lead nitrate showed remarkable results. We observed an increase in the activity of SOD, GR, and GPx, coupled with a decrease in CAT activity. In addition, GPx activity was increased in the HR rats when L-NNA was administered prior to the lead nitrate exposure. In particular, significant improvements in GPx activity were observed following the administration of L-arginine to the LR animals both before and after the exposure to lead nitrate.
Investigations of the effects of L-arginine and L-NNA administered before and after exposure to lead nitrate may provide potential strategies for the prevention and treatment of lead poisoning. Understanding these effects, which depend on individual resistance to hypoxia, is crucial for the development of effective interventions. Understanding the balance between the beneficial and harmful effects of NO, especially in such stressful conditions as hypoxia and toxic exposure, is essential for safe therapeutic applications, as NO has multiple roles in physiological processes.
Conclusion
Our study focused on evaluating mitochondrial oxygen consumption, microsomal oxidation, intensity of lipoperoxidation processes and antioxidant defences in the liver of rats with low and high resistance to hypoxia to elucidate the mechanisms of L-arginine and L-NNA effects during lead nitrate exposure. The efficient redistribution of metabolic pathways involving oxygen-dependent processes is a characteristic feature of animals with high resistance (HR) to hypoxia. This redistribution towards mitochondrial oxidative processes under the influence of the nitric oxide precursor amino acid L-arginine is crucial for maintaining mitochondrial respiratory chain function, especially as mitochondrial complex activity is significantly altered under lead nitrate exposure.
Our experiments showed a significant increase in lead levels in liver tissue compared to the control group. Lead nitrate significantly reduced nitrite and nitrate levels, stable metabolic components of the nitric oxide system, in both low resistance (LR) and high resistance (HR) animals. L-arginine effectively attenuated these effects in both groups, resulting in statistically significant changes. In addition, HR rats showed a significant increase in nitrate levels compared to the lead nitrate-exposed group. Mitochondrial energy supply processes were less efficient, particularly in LR rats, which showed greater susceptibility to NADH-dependent oxidation during lead nitrate exposure. L-arginine administration induced oxidation of NADH-dependent substrates, primarily α-ketoglutarate compared to succinate, in LR animals. This directional effect was more pronounced when the nitric oxide system was reduced with L-NNA in HR rats.
Changes in antioxidant enzyme activities were observed in rat liver tissue exposed to lead nitrate, altering the catalase-peroxidase ratio. Responses in SOD, CAT, GPx and GR activities varied due to lead nitrate exposure and their activation mechanisms. L-arginine significantly increased GPx activity in LR rats before and after lead nitrate exposure. This study could advance our understanding of how lead nitrate interacts with protective mechanisms and how it affects physiological reactivity, potentially improving L-arginine-based therapeutic strategies to mitigate the effects of lead nitrate exposure. Further research into the mechanisms and protective substances of lead is essential to understand and mitigate its toxicity.
Acknowledgements
The present study was financially supported by the Pomeranian University in Słupsk and T.H. Shevchenko National University “Chernihiv Colehium”, Chernihiv, Ukraine. The authors would like to thank the Pomeranian University in Słupsk and T.H. Shevchenko National University “Chernihiv Colehium” for supporting this research.
Authors’ contributions
The authors contributed to the following aspects of the study Conceptualisation: HT, NK, OL, PK; Data curation: HT, NK; Formal analysis: HT, NK; Investigation: HT, NK, OL; Methodology: HT, NK; Supervision: NK, HT; Writing – original draft: NK, HT; Writing – revision and editing: HT, NK, PK.
Halina Tkaczenko, ORCID: https://orcid.org/0000-0003-3951-9005;
Oleksandr Lukash, ORCID: https://orcid.org/0000-0003-2702-6430;
Piotr Kamiński, ORCID: https://orcid.org/0000-0003-1978-6018;
Natalia Kurhaluk, ORCID: https://orcid.org/0000-0002-4669-1092.
Funding
The present study was financially supported by the Pomeranian University in Słupsk (Słupsk, Poland) and T.H. Shevchenko National University “Chernihiv Colehium”, Chernihiv, Ukraine.
Disclosure Statement
The authors have no competing interests to declare.
References
| 1 | Assi MA, Hezmee MN, Haron AW, Sabri MY, Rajion MA. The detrimental effects of lead on human and animal health. Vet World 2016;9:660-671.
https://doi.org/10.14202/vetworld.2016.660-671 |
| 2 | Karp RJ. Redlining and Lead Poisoning: Causes and Consequences. J Health Care Poor Underserved 2023;34:431-446.
https://doi.org/10.1353/hpu.2023.0028 |
| 3 | Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease - a systematic review. Environ Health Perspect 2007;115:472-482.
https://doi.org/10.1289/ehp.9785 |
| 4 | Rastogi SK. Renal effects of environmental and occupational lead exposure. Indian J Occup Environ Med 2008;12:103-106.
https://doi.org/10.4103/0019-5278.44689 |
| 5 | Mason LH, Harp JP, Han DY. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int 2014;2014:840547.
https://doi.org/10.1155/2014/840547 |
| 6 | Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int J Mol Sci 2015;16:29592-29630.
https://doi.org/10.3390/ijms161226183 |
| 7 | Ramírez Ortega D, González Esquivel DF, Blanco Ayala T, Pineda B, Gómez Manzo S, Marcial Quino J, Carrillo Mora P, Pérez de la Cruz V. Cognitive Impairment Induced by Lead Exposure during Lifespan: Mechanisms of Lead Neurotoxicity. Toxics 2021;9:23.
https://doi.org/10.3390/toxics9020023 |
| 8 | Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020;6:e04691.
https://doi.org/10.1016/j.heliyon.2020.e04691 |
| 9 | Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front Pharmacol 2021;12:643972.
https://doi.org/10.3389/fphar.2021.643972 |
| 10 | Dabrowska-Bouta B, Struzyńska L, Rafałowska U. Effect of acute and chronic lead exposure on the level of sulfhydryl groups in rat brain. Acta Neurobiol Exp (Wars) 1996;56:233-236.
https://doi.org/10.55782/ane-1996-1125 |
| 11 | Jurczuk M, Moniuszko-Jakoniuk J, Brzóska MM. Involvement of some low-molecular thiols in the peroxidative mechanisms of lead and ethanol action on rat liver and kidney. Toxicology 2006;219:11-21.
https://doi.org/10.1016/j.tox.2005.10.022 |
| 12 | Virgolini MB, Aschner M. Molecular mechanisms of lead neurotoxicity. Adv Neurotoxicol 2021;5:159-213.
https://doi.org/10.1016/bs.ant.2020.11.002 |
| 13 | Bandaru LJM, Murumulla L, CBL, DKP, Challa S. Exposure of combination of environmental pollutant, lead (Pb) and β-amyloid peptides causes mitochondrial dysfunction and oxidative stress in human neuronal cells. J Bioenerg Biomembr 2023;55:79-89.
https://doi.org/10.1007/s10863-023-09956-9 |
| 14 | Lee JW, Choi H, Hwang UK, Kang JC, Kang YJ, Kim KI, Kim JH. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ Toxicol Pharmacol 2019;68:101-108
https://doi.org/10.1016/j.etap.2019.03.010 |
| 15 | Sharma S, Raghuvanshi BP, Shukla S. Toxic effects of lead exposure in rats: involvement of oxidative stress, genotoxic effect, and the beneficial role of N-acetylcysteine supplemented with selenium. J Environ Pathol Toxicol Oncol 2014;33:19-32.
https://doi.org/10.1615/JEnvironPatholToxicolOncol.2014009712 |
| 16 | Hsu PC, Guo YL. Antioxidant nutrients and lead toxicity. Toxicology 2002;180:33-44.
https://doi.org/10.1016/S0300-483X(02)00380-3 |
| 17 | Gillis BS, Arbieva Z, Gavin IM. Analysis of lead toxicity in human cells. BMC genomics 2012;13:344.
https://doi.org/10.1186/1471-2164-13-344 |
| 18 | Metryka E, Chibowska K, Gutowska I, Falkowska A, Kupnicka P, Barczak K, Chlubek D, Baranowska-Bosiacka I. Lead (Pb) Exposure Enhances Expression of Factors Associated with Inflammation. Int J Mol Sci 2018;19:1813.
https://doi.org/10.3390/ijms19061813 |
| 19 | Renu K, Chakraborty R, Myakala H, Koti, R, Famurewa AC, Madhyastha H, Vellingiri B, George A, Valsala Gopalakrishnan A. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium) - induced hepatotoxicity - A review. Chemosphere 2021;271:129735.
https://doi.org/10.1016/j.chemosphere.2021.129735 |
| 20 | Teschke R. Aluminum, Arsenic, Beryllium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Mercury, Molybdenum, Nickel, Platinum, Thallium, Titanium, Vanadium, and Zinc: Molecular Aspects in Experimental Liver Injury. Int J Mol Sci 2022;23:12213.
https://doi.org/10.3390/ijms232012213 |
| 21 | Wagner PJ, Park HR, Wang Z, Kirchner R, Wei Y, Su L, Stanfield K, Guilarte TR, Wright RO, Christiani DC, Lu Q. In vitro Effects of Lead on Gene Expression in Neural Stem Cells and Associations between Up-regulated Genes and Cognitive Scores in Children. Environ Health Perspect 2017;125:721-729.
https://doi.org/10.1289/EHP265 |
| 22 | Park HR, Azzara D, Cohen ED, Boomhower SR, Diwadkar AR, Himes BE, O'Reilly MA, Lu Q. Identification of novel NRF2-dependent genes as regulators of lead and arsenic toxicity in neural progenitor cells. J Hazard Mater 2024;463:132906.
https://doi.org/10.1016/j.jhazmat.2023.132906 |
| 23 | Rodríguez J, Mandalunis PM. A Review of Metal Exposure and Its Effects on Bone Health. J Toxicol 2018;2018:4854152.
https://doi.org/10.1155/2018/4854152 |
| 24 | Pounds JG, Long GJ, Rosen JF. Cellular and molecular toxicity of lead in bone. Environ Health Perspect 1991;91:17-32.
https://doi.org/10.1289/ehp.919117 |
| 25 | Sakai T. Biomarkers of lead exposure. Ind Health 2000;38:127-142.
https://doi.org/10.2486/indhealth.38.127 |
| 26 | El-Sheikh NM, Khalil FA. L-arginine and L-glutamine as immunonutrients and modulating agents for oxidative stress and toxicity induced by sodium nitrite in rats. Food Chem Toxicol 2011;49:758-762.
https://doi.org/10.1016/j.fct.2010.11.039 |
| 27 | Li M, Qin J, Xiong K, Jiang B, Zhang T. Review of arginase as a promising biocatalyst: characteristics, preparation, applications and future challenges. Crit Rev Biotechnol 2022;42:651-667.
https://doi.org/10.1080/07388551.2021.1947962 |
| 28 | Kurhaluk N. Tricarboxylic Acid Cycle Intermediates and Individual Ageing. Biomolecules 2024;14:260.
https://doi.org/10.3390/biom14030260 |
| 29 | Szlas A, Kurek JM, Krejpcio Z. The Potential of L-Arginine in Prevention and Treatment of Disturbed Carbohydrate and Lipid Metabolism - A Review. Nutrients 2022;14:961.
https://doi.org/10.3390/nu14050961 |
| 30 | Kurhaluk N. The Effectiveness of L-arginine in Clinical Conditions Associated with Hypoxia. Int J Mol Sci 2023a;24:8205.
https://doi.org/10.3390/ijms24098205 |
| 31 | Kurhaluk N. Supplementation with l-arginine and nitrates vs age and individual physiological reactivity. Nutr Rev 2023b;nuad131. Advance online publication. https://doi.org/10.1093/nutrit/nuad131.
https://doi.org/10.1093/nutrit/nuad131 |
| 32 | Kurhaluk N, Tkachenko H, Lukash O. Photoperiod-induced alterations in biomarkers of oxidative stress and biochemical pathways in rats of different ages: Focus on individual physiological reactivity. Chronobiol Int 2021;38:1673-1691.
https://doi.org/10.1080/07420528.2021.1939364 |
| 33 | Kurhaluk N, Lukash O, Tkaczenko H. Do the Effects of Krebs Cycle Intermediates on Oxygen-Dependent Processes in Hypoxia Mediated by the Nitric Oxide System Have Reciprocal or Competitive Relationships?. Cell Physiol Biochem 2023c;57:426-451.
https://doi.org/10.33594/000000669 |
| 34 | Tkachenko H, Kurhalyuk N, Khabrovska L, Kamiński P. Effect of L-arginine on lead induced oxidative stress in the blood of rats with different resistance to hypoxia. Pol J Food Nutr Sci 2007;57:387-394.
|
| 35 | Tkachenko H, Kurhalyuk N. Role of L-arginine Against Lead Toxicity in Liver of Rats with Different Resistance to Hypoxia. Pol J Environ Stud 2011;20:1319-1325.
|
| 36 | Kurhaliuk NM. Stan mitokhondrial'noho dykhannia i kal'tsiievoï iemnosti pechinky shchuriv z riznoiu rezystentnistiu do hipoksiï za umov vvedennia L-arhininu [State of mitochondrial respiration and calcium capacity in livers of rats with different resistance to hypoxia after injections of L-arginine]. Fiziol Zh (1994) 2001;47:64-72.
|
| 37 | Kurhaluk N, Lukash O, Nosar V, Portnychenko A, Portnichenko V, Wszedybyl-Winklewska M, Winklewski PJ. Liver mitochondrial respiratory plasticity and oxygen uptake evoked by cobalt chloride in rats with low and high resistance to extreme hypobaric hypoxia. Can J Physiol Pharmacol 2019;97:392-399.
https://doi.org/10.1139/cjpp-2018-0642 |
| 38 | Belosludtsev KN, Dubinin MV, Talanov EY, Starinets VS, Tenkov KS, Zakharova NM, Belosludtseva NV. Transport of Ca2+ and Ca2+-Dependent Permeability Transition in the Liver and Heart Mitochondria of Rats with Different Tolerance to Acute Hypoxia. Biomolecules 2020;10:114.
https://doi.org/10.3390/biom10010114 |
| 39 | Dzhalilova DS, Diatroptov ME, Tsvetkov IS, Makarova OV, Kuznetsov SL. Expression of Hif-1α, Nf-κb, and Vegf Genes in the Liver and Blood Serum Levels of HIF-1α, Erythropoietin, VEGF, TGF-β, 8-Isoprostane, and Corticosterone in Wistar Rats with High and Low Resistance to Hypoxia. Bull Exp Biol Med 2018;165:781-785.
https://doi.org/10.1007/s10517-018-4264-x |
| 40 | Dzhalilova D, Kosyreva A, Lokhonina A, Tsvetkov I, Vishnyakova P, Makarova O, Fatkhudinov T. Molecular and phenotypic distinctions of macrophages in tolerant and susceptible to hypoxia rats. PeerJ 2023a;11:e16052.
https://doi.org/10.7717/peerj.16052 |
| 41 | Dzhalilova DS, Silina MV, Kosyreva AM, Tsvetkov IS, Makarova OV. Morphological and Molecular Biological Features of the Systemic Inflammatory Response in Old Wistar Rats with High and Low Resistance to Hypoxia. Bull Exp Biol Med 2023b;175:704-710.
https://doi.org/10.1007/s10517-023-05930-y |
| 42 | Kosyreva AM, Dzhalilova DS, Tsvetkov IS, Makarova MA, Makarova OV. Ex Vivo Production of IL-1β and IL-10 by Activated Blood Cells of Wistar Rats with Different Resistance to Hypoxia after Systemic Inflammatory Response Syndrome. Bull Exp Biol Med 2023;176:290-296.
https://doi.org/10.1007/s10517-024-06010-5 |
| 43 | Bayanov AA, Brunt AR. Role of hypoxia and constitutionally different resistance to hypoxia/stress as the determiners of individual profile of cytochrome P450 isozyme activity. Gen Pharmacol 1999;33:355-361.
https://doi.org/10.1016/S0306-3623(98)00288-2 |
| 44 | Lukyanova L, Germanova E, Khmil N, Pavlik L, Mikheeva I, Shigaeva M, Mironova G. Signaling Role of Mitochondrial Enzymes and Ultrastructure in the Formation of Molecular Mechanisms of Adaptation to Hypoxia. Int J Mol Sci 2021;22:8636.
https://doi.org/10.3390/ijms22168636 |
| 45 | Dzhalilova DS, Kosyreva AM, Diatroptov ME, Ponomarenko EA, Tsvetkov IS, Zolotova NA, Mkhitarov VA, Khochanskiy DN, Makarova OV. Dependence of the severity of the systemic inflammatory response on resistance to hypoxia in male Wistar rats. J Inflamm Res 2019a;12:73-86.
https://doi.org/10.2147/JIR.S194581 |
| 46 | Dzhalilova DS, Kosyreva AM, Diatroptov ME, Zolotova NA, Tsvetkov IS, Mkhitarov VA, Makarova OV, Khochanskiy DN. Morphological Characteristics of the Thymus and Spleen and the Subpopulation Composition of Lymphocytes in Peripheral Blood during Systemic Inflammatory Response in Male Rats with Different Resistance to Hypoxia. Int J Inflam 2019b;2019:7584685.
https://doi.org/10.1155/2019/7584685 |
| 47 | Mironova GD, Shigaeva MI, Gritsenko EN, Murzaeva SV, Gorbacheva OS, Germanova EL, Lukyanova LD. Functioning of the mitochondrial ATP-dependent potassium channel in rats varying in their resistance to hypoxia. Involvement of the channel in the process of animal's adaptation to hypoxia. J Bioenerg Biomembr 2010;42:473-481.
https://doi.org/10.1007/s10863-010-9316-5 |
| 48 | Kondrashova MN, Doliba NM. Polarographic observation of substrate-level phosphorylation and its stimulation by acetylcholine. FEBS Lett 1989;243:153-155.
https://doi.org/10.1016/0014-5793(89)80119-X |
| 49 | Wisniewski JA, Moody DE, Hammock BD, Shull LR. Interlobular distribution of hepatic xenobiotic-metabolizing enzyme activities in cattle, goats and sheep. J Anim Sci 1987;64:210-215.
https://doi.org/10.2527/jas1987.641210x |
| 50 | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-254.
https://doi.org/10.1016/0003-2697(76)90527-3 |
| 51 | Tkachenko H, Kurhaluk N, Kasiyan O, Kamiński P. Dietary nutrients and health risks from exposure to some heavy metals through the consumption of the farmed common carp (Cyprinus carpio). J Environ Health Sci Eng 2021;19:793-804.
https://doi.org/10.1007/s40201-021-00647-4 |
| 52 | Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 1955a;217:383-393.
https://doi.org/10.1016/S0021-9258(19)57189-7 |
| 53 | Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem 1955b;217:409-427.
https://doi.org/10.1016/S0021-9258(19)57191-5 |
| 54 | Chance B. Reaction of oxygen with the respiratory chain in cells and tissues. J Gen Physiol 1965;49:163-195.
https://doi.org/10.1085/jgp.49.1.163 |
| 55 | Kamyshnikov VS. Reference book on clinic and biochemical researches and laboratory diagnostics. MEDpress-inform, Moscow, 2004.
|
| 56 | Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978;52:302-310.
https://doi.org/10.1016/S0076-6879(78)52032-6 |
| 57 | Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 1994;233:357-363.
https://doi.org/10.1016/S0076-6879(94)33041-7 |
| 58 | Kostiuk VA, Potapovich AI, Kovaleva ZhV. Prostoĭ i chuvstvitel'nyĭ metod opredeleniia aktivnosti superoksid-dismutazy, osnovannyĭ na reaktsii okisleniia kvertsetina [A simple and sensitive method of determination of superoxide dismutase activity based on the reaction of quercetin oxidation]. Vopr Med Khim 1990;36:88-91.
|
| 59 | Koroliuk MA, Ivanova LI, Maĭorova IG, Tokarev VE. Metod opredeleniia aktivnosti katalazy [A method of determining catalase activity]. Lab delo 1988;:16-19.
|
| 60 | Glatzle D, Vuilleumier JP, Weber F, Decker K. Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientia 1974;30:665-667.
https://doi.org/10.1007/BF01921531 |
| 61 | Moin V. M. Prostoĭ i spetsificheskiĭ metod opredeleniia aktivnosti glutationperoksidazy v éritrotsitakh [A simple and specific method for determining glutathione peroxidase activity in erythrocytes]. Lab. delo 1986;:724-727.
|
| 62 | Galaktionova LP, Molchanov AV, El'chaninova SA, Varshavskiĭ Bia. Sostoianie perekisnogo okisleniia u bol'nykh s iazvennoĭ bolezn'iu zheludka i dvenadtsatiperstnoĭ kishki [Lipid peroxidation in patients with gastric and duodenal peptic ulcers]. Klin Lab Diagn 1998;:10-14.
|
| 63 | Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1982;126:131-138.
https://doi.org/10.1016/0003-2697(82)90118-X |
| 64 | Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 1994;356:295-298.
https://doi.org/10.1016/0014-5793(94)01290-3 |
| 65 | Karuzina II, Archakov AI. Estimation of the microsomal fraction of the liver and characteristic of its oxidative systems. Modern methods in biochemistry. Ed. V.I. Orekhovich. Moscow, Medicine, 1977,p.49-52.
|
| 66 | Matsubara T, Touchi A, Tochino Y. Hepatic aminopyrine N-demethylase system: further studies of assay procedure. Jpn J Pharmacol 1977;27:127-136.
https://doi.org/10.1254/jjp.27.127 |
| 67 | Zar JH. Biostatistical Analysis. 4th ed., Prentice Hall Inc., New Jersey, 1999.
|
| 68 | Lu SC. Regulation of glutathione synthesis. Mol Aspects Med 2009;30:42-59.
https://doi.org/10.1016/j.mam.2008.05.005 |
| 69 | Lu SC. Glutathione synthesis. Biochim Biophys Acta 2013;1830:3143-3153.
https://doi.org/10.1016/j.bbagen.2012.09.008 |
| 70 | Devóz PP, Reis MBD, Gomes WR, Maraslis FT, Ribeiro DL, Antunes LMG, Batista BL, Grotto D, Reis RM, Barbosa FJr, Barcelos GRM. Adaptive epigenetic response of glutathione (GSH)-related genes against lead (Pb)-induced toxicity, in individuals chronically exposed to the metal. Chemosphere 2021;269:128758.
https://doi.org/10.1016/j.chemosphere.2020.128758 |
| 71 | Star RA. Nitric oxide. Am J Med Sci 1993;306:348-358.
https://doi.org/10.1097/00000441-199311000-00015 |
| 72 | Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS. Nitric Oxide and Endothelial Dysfunction. Crit Care Clin 2020;36:307-321.
https://doi.org/10.1016/j.ccc.2019.12.009 |
| 73 | Lundberg JO, Weitzberg E. Nitric oxide signaling in health and disease. Cell 2022;185:2853-2878.
https://doi.org/10.1016/j.cell.2022.06.010 |
| 74 | Oral O. Nitric oxide and its role in exercise physiology. J Sports Med Phys Fitness 2021;61:1208-1211.
https://doi.org/10.23736/S0022-4707.21.11640-8 |
| 75 | Kiani AK, Bonetti G, Medori MC, Caruso P, Manganotti P, Fioretti F, Nodari S, Connelly ST, Bertelli M. Dietary supplements for improving nitric-oxide synthesis. J Prev Med Hyg 2022;63:239-E245.
|
| 76 | Fujii J, Yamada KI. Defense systems to avoid ferroptosis caused by lipid peroxidation-mediated membrane damage. Free Radic Res 2023;57:353-372.
https://doi.org/10.1080/10715762.2023.2244155 |
| 77 | Dios-Barbeito S, González R, Cadenas M, García LF, Victor VM, Padillo FJ, Muntané J. Impact of nitric oxide in liver cancer microenvironment. Nitric Oxide 2022;128:1-11.
https://doi.org/10.1016/j.niox.2022.07.006 |
| 78 | Shaban NZ, Abdelrahman SA, El-Kersh MAL, Mogahed FAK, Talaat IM, Habashy NH. The synergistic hepatoprotective potential of Beta vulgaris juice and 2, 3-dimercaptosuccinic acid in lead-intoxicated rats via improving the hepatic oxidative and inflammatory stress. BMC Complement Med Ther 2020;20:268.
https://doi.org/10.1186/s12906-020-03056-6 |
| 79 | Poeggeler B, Singh SK, Sambamurti K, Pappolla MA. Nitric Oxide as a Determinant of Human Longevity and Health Span. Int J Mol Sci 2023;24:14533.
https://doi.org/10.3390/ijms241914533 |
| 80 | Wu J, Jia J, Ji D, Jiao W, Huang Z, Zhang Y. Advances in nitric oxide regulators for the treatment of ischemic stroke. Eur J Med Chem 2023;262:115912.
https://doi.org/10.1016/j.ejmech.2023.115912 |
| 81 | Haswell-Elkins MR, Satarug S, Tsuda M, Mairiang E, Esumi H, Sithithaworn P, Mairiang P, Saitoh M, Yongvanit P, Elkins DB. Liver fluke infection and cholangiocarcinoma: model of endogenous nitric oxide and extragastric nitrosation in human carcinogenesis. Mutat Res 1994;305:241-252.
https://doi.org/10.1016/0027-5107(94)90244-5 |
| 82 | Carmignani M, Volpe AR, Boscolo P, Qiao N, Di Gioacchino M, Grilli A, Felaco M. Catcholamine and nitric oxide systems as targets of chronic lead exposure in inducing selective functional impairment. Life Sci 2000;68:401-415.
https://doi.org/10.1016/S0024-3205(00)00954-1 |
| 83 | Lukyanova LD, Dudchenko AM, Tsybina TA, Germanova EL, Tkachuk EN, Erenburg IV. Effect of intermittent normobaric hypoxia on kinetic properties of mitochondrial enzymes. Bull Exp Biol Med 2007;144:795-801.
https://doi.org/10.1007/s10517-007-0434-y |
| 84 | Luk'yanova LD, Dudchenko AM. Parameters of adenylate pool as predictors of energy metabolism disturbances in hepatocytes during hypoxia. Bull Exp Biol Med 2003;136:34-37.
https://doi.org/10.1023/A:1026028627420 |
| 85 | Zhang H, Sun H, Peng A, Guo S, Wang M, Loor JJ, Wang H. N-carbamylglutamate and l-arginine promote intestinal function in suckling lambs with intrauterine growth restriction by regulating antioxidant capacity via a nitric oxide-dependent pathway. Food Funct 2019;10:6374-6384.
https://doi.org/10.1039/C9FO01752F |
| 86 | Masri FA, Comhair SA, Koeck T, Xu W, Janocha A, Ghosh S, Dweik RA, Golish J, Kinter M, Stuehr DJ, Erzurum SC, Aulak KS. Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med 2005;172:597-605.
https://doi.org/10.1164/rccm.200411-1523OC |
| 87 | Förstermann U, Xia N, Li H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ Res 2017;120:713-735.
https://doi.org/10.1161/CIRCRESAHA.116.309326 |
| 88 | Galkin A, Higgs A, Moncada S. Nitric oxide and hypoxia. Essays Biochem 2007;43:29-42.
https://doi.org/10.1042/bse0430029 |
| 89 | Stewart VC, Sharpe MA, Clark JB, Heales SJ. Astrocyte-derived nitric oxide causes both reversible and irreversible damage to the neuronal mitochondrial respiratory chain. J Neurochem 2000;75:694-700.
https://doi.org/10.1046/j.1471-4159.2000.0750694.x |
| 90 | Radi R, Cassina A, Hodara R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem 2002;383:401-409.
https://doi.org/10.1515/BC.2002.044 |
| 91 | Brown GC, Borutaite V. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc Res 2007;75:283-290.
https://doi.org/10.1016/j.cardiores.2007.03.022 |