The Effect of Oleuropein in AIF and MMP-9 in Traumatic Brain Injury Rat Model
bDepartment of Pharmacy, Pharmacy Faculty, Universitas Sumatera Utara, Medan, Indonesia,
cDepartment of Neurology, Medical Faculty, Universitas Sumatera Utara, Medan, Indonesia,
dDepartment of Neurosurgery, Medical Faculty, Universitas Indonesia, Jakarta, Indonesia,
eDepartment of Biology, Mathematic and Natural Sciences Faculty, Universitas, Sumatera Utara, Medan, Indonesia,
fDepartment of Clinical Pathology, Medical Faculty, Universitas Sumatera, Utara, Medan, Indonesia,
gDepartment of Biochemistry and Biology Molecular, Medical Faculty, Universitas Brawijaya, Malang, Indonesia
Keywords
Abstract
Background/Aims:
Traumatic brain injury is a significant public problem with an incidence of 10 million people per year, causing the largest deaths and disabilities worldwide. Head injuries can be classified into primary and secondary head injuries. Secondary head injuries can be caused by several factors such as ischemia, cerebral edema, and neuroinflammation. AIF and MMP-9 are two parameters that can be indicators in measuring the effect of Oleuropein on traumatic brain injury in rats. Oleuropein itself has many activities such as antioxidant, anti-apoptotic, antimicrobial, anti-inflammatory, and neuroprotective.Methods:
Adult male Sprague-Dawley rats (250-350 grams) were exposed to head injury, with or without intraperitoneal administration of Oleuropein. Within 24-72 hours brain tissue was isolated for immunohistochemical analysis, ELISA, and TUNEL. AIF, GFAP, MMP-9, and HMGB-1 levels were determined using immunohistochemistry in both the control and treatment groups. Statistical analysis was made using the One-Way Analysis of Variance (ANOVA) and paired t-test.Results:
The results showed that Oleuropein was able to reduce AIF and MMP-9 levels in rats with traumatic brain injury. This indicates that Oleuropein has a neuroprotective effect by reducing inflammation and apoptosis.Conclusion:
Oleuropein has a potential neuroprotective effect in traumatic brain injury by reducing inflammation and apoptosis. Therefore, Oleuropein can be considered as a potential therapeutic agent for traumatic brain injury in the future.Introduction
Head injury is a significant global public health problem and is often referred to as a “silent epidemic”. It is the leading cause of death and disability due to injury worldwide, resulting from external impact that causes changes in neurological function or pathological lesions in the brain. Globally, around 57 million people have been hospitalized with one or more traumatic brain injury, and approximately 10 million people worldwide are affected by head injuries each year. The incidence of head injuries worldwide is 250 cases per 100, 000 population, in which falling from heights, traffic accidents, and physical violence being the primary causes of head injuries in developing countries [1-3].
In 2014, the US witnessed approximately 2.5 million head injuries, encompassing incidents leading to emergency room visits, hospitalizations, and fatalities, including among children. European data highlights that 37% of fatal injury cases are attributed to head injuries. Those with low socio-economic status and military personnel face a heightened susceptibility to head injuries. Globally, head injuries contribute to the highest disability rates, impacting an estimated 13 million individuals in Europe and the US. Furthermore, 10-15% of head injury patients require neurosurgery, with severe cases receiving treatment in intensive care [4, 5].
Head injuries are relatively prevalent in Indonesia with an incidence rate of 6-12% and a mortality rate of 23-37%. According to the Ministry of Health in 2012, these injuries rank among the top ten diseases treated in hospitals. In 2020, HAM Medan Hospital’s Neurosurgery Department treated 343 patients with head injuries in the Emergency Room, including 232 cases of mild head injury, 61 cases of moderate head injury, and 50 cases of severe head injury. Head injuries are classified as primary or secondary, with primary head injury resulting from direct trauma and causing superficial or deeper lesions due to the acceleration, deceleration, and rotational mechanisms of the injury [4, 5].
Apoptosis can be induced through several pathways in cases of head injury. Werner identified two pathways of apoptosis: intrinsic and extrinsic. The B-cell lymphoma-2 pathway contains components that are both pro-apoptotic and anti-apoptotic. The BH-3 protein acts as a connector between the extrinsic pathway and mitochondrial apoptosis, and is cleaved by caspase-8, which increases the permeability of the mitochondrial membrane. Upon activation of apoptosis signals, caspase-9 is amplified, serving as an initiator caspase that subsequently activates caspase-3, initiating the apoptosis process. Additionally, the caspase-independent pathway is influenced by AIF, which translocates to the nucleus during the pro-apoptotic process and activates DNA degradation. Cheng has stated that AIF expression is sufficient to cause cell death [6, 7].
MMP-9 is the most common type of Matrix Metalloproteinase activated during ischemia. It disrupts the blood-brain barrier critical shield safeguarding the brain from detrimental substances. Reducing MMP-9 expression can prevent neuronal cell death and neurological deficits. NF-κB triggers an inflammatory cascade that regulates MMP-9 transcription. Elevated MMP levels, prompted by inflammatory cells, trigger apoptosis activation. MMP-9 levels peak at 24-72 hours after severe head injury, exhibiting a negative correlation with patient prognosis. MMP-9 can be used as a rescue therapy for head injury [8-11].
Oleuropein, found in olives, possesses beneficial properties such as antioxidant activity, anti-inflammatory effects, and neuroprotection, aiding cell survival and diminishing apoptosis. In ischemic conditions, oleuropein proves effective by reducing infarct volume, cerebral edema, and improving neurological deficits. Additionally, the administration of citicoline enhances outcomes in traumatic brain injury patients, impacting brain edema, consciousness, electroencephalography changes, and overall quality of life. Nevertheless, notable differences exist between animal and clinical studies regarding endpoints and treatment methods, as no animal model can fully replicate all pathological changes observed in humans [12-16]. The aim of this study is to analyze the effect of Oleuropein administration on rats with a traumatic brain injury model using AIF and MMP-9 parameters.
Materials and Methods
A true experimental study was performed with random sampling technique to ensure homogenous sample. This experiment used pre-test and post-test control group design by comparing intervention effects on experimental group and control group [17].
Animals
This research has received ethical approval from the Ethical Committee for Animal Research, as indicated by the approval letter with reference number 1174/KEP/USU/2021. This evidence of approval by an animal ethics committee was based on the Nuremberg Code and Helsinki declaration.
The animal used in this study is adult male Sprague-Dawley rat. The inclusion criteria for this study are vitality and activity, aged 2.25 months, total weight of 250-350 grams. A total sample of 30 rats were included in this study and divided through simple randomization process to ensure non biased results. Rats were acclimatized to the laboratory conditions for a week before the commencement of the experiment.
The samples were divided into six groups: Group A (negative control without TBI+ placebo), Group B (positive control with TBI + placebo), Group C (TBI + oleuropein 50mg/kgBB), Group D (TBI + oleuropein 100mg/kgBB), Group E (TBI + oleuropein 50mg/kgBB pre and post-treatment), and Group F (TBI + oleuropein 50mg/kgBB pre and post-treatment). The control groups (group A and B) were injected with placebo to compare the effectivity of Oleuropein against TBI. The negative control group (group A) received sham treatment without Marmarou’s weight drop to compare the effect of TBI on cell numbers.
The experimental groups received treatment on the first day. Group C received TBI induction and 50 mg/kgBB Oleuropein injection after TBI induction. Group D received TBI induction and 100 mg/kgBB Oleuropein injection after TBI induction. To measure the protective properties of oleuropein, injection was done before and after TBI induction. Group E was injected with 50 mg/kgBB Oleuropein before and after TBI induction and Group F was injected with 100 mg/kgBB Oleuropein before and after TBI induction.
TBI Induction
The rats were anesthetized with ether (Drager Narkomed Ventilator, US). The anesthesia is administered with an initial gas volume dose of 200-250 ml, at a rate of 15 cycles per minute, using a mixture of nitrous oxide (N2O) at 70% and oxygen (O2) at 30%. The percentage of administered isoflurane is adjusted using the “dial” on the Drager 19.1 Isoflurane Vaporizer, ranging from 0.5% to 4.0%. Anesthesia is administered during the surgical period through an endotracheal intubation tube and the animal is provided with mechanical ventilation or a transfer system with a Cone-Mask.
TBI induction in this study was performed using Marmarou’s weight drop model. The biomechanics of this model enable the replication of TBI severity by adjusting the height (1-2.1 m) and weight (500 or 450 g) dropped. For mild injury, 450 g of weight was dropped from a height of 1 m; for moderate injury, 450 g of weight was dropped from a height of 2 m; for severe injury, 500 g of weight was dropped from a height of 2.1 m. Secondary attacks of hypoxia and hypotension were induced by manipulating anesthesia level and administration, immediately after traumatic brain injury, and maintained based on the experimental design for a period of 10-30 minutes post-injury [13].
Oleuropein Injection
Different doses of Oleuropein 98% (Sigma Aldrich) were used to compare different effects on TBI. Oleuropein powder (Sigma-Aldrich) was dissolved in normal saline, and injected intraperitoneally. Oleuropein was injected right after TBI induction for group C and D, but it was done before and after TBI induction for group E and F. The dose of Oleuropein according to previous literature using animal models with the best neuroprotective effect starts at 30 mg/kg.16 We compared the Oleuropein dosing of 50 mg/kgBB and 100 mg/kgBB post TBI induction in group C and D. We also measured the protective properties of Oleuropein by giving injections pre and post TBI induction in 50 mg/kgBB and 100 mg/kgBB (group E andF) [14, 15].
Observation
The study utilized pre and post-test measurements to assess the effects of oleuropein injection on TBI. Observations were conducted after the initial 24 hours and continued for the subsequent 72 hours. AIF, GFAP, HMGB-1, and MMP-9 levels increased within the first 24 hours of head injury onset. All rats in control and treatment groups were sacrificed on day 3 by cervical dislocation after anesthesia with ether [18].
Histopathological asessment
Brain samples were obtained and fixed in 10% formaldehyde solution and embedded in paraffin. The coronal sections (5 µm) of the the cerebral cortex or sagittal sections of the brainstem were selected randomly using a microtome. For histopathological assessment, some tissue sections were deparaffinized with xylene, stained with hematoxylin-eosin and multiplex immunohistochemistry (IHC) protocol. Samples were used for immunohistochemical, ELISA, and TUNEL coloring and examinations. Samples were analyzed with 1000X light microscope, with positive cells counted in 20 fields of view (HPF) in each sample.
Immunohistochemistry
Immunohistochemistry examinations were done by incubating the sections with goat serum (in order to block nonspecific site), polyclonal rabbit anti-Bax antibody, or anti-Bcl-2 rabbit polyclonal antibody at 4°C overnight. The sections were then washed with PBS and incubated with secondary antibody conjugated with horseradish peroxidase (goat anti-rabbit IgG, Abcam, USA) for 2 hours and detected by diaminobenzidine tetrahydrochloride for 5 minutes. Afterwards, they were dehydrated and mounted. For negative controls, primary antibodies were omitted. For quantitative analysis, immunohistochemical photographs (10 photos from each sample collected from all rats in each experimental group) were assessed by densitometry using MacBiophotonics Image J 1.41a software on a personal computer.
TUNEL Assay
The TUNEL (Terminal deoxynucleotidyl transferase dUTP Nick End Labeling-) assay was performed to assess DNA fragmentation, a hallmark of apoptotic cell death. Fixed and permeabilized cells or tissue sections were incubated with the TUNEL reaction mixture. Counterstaining with hematoxylin facilitated morphological visualization. The samples were mounted and examined microscopically. Controls and optimizations were implemented according to kit instructions (Abcam kit with Batch Number BrdU-Red ab66110), ensuring the reliability of the assay in detecting apoptotic events.
ELISA
ELISA was done using the sandwich method, a precise and widely utilized technique, involving specific reagents for the detection of key biomarkers. The ELISA assay was done using a commercial kit (Abcam kit ab181421). The selected biomarkers for analysis included Apoptosis Inducing Factor (AIF), Glial Acidic Fibrillary Protein (GAFP), Matrix Metalloproteinase-9 (MMP-9), and High Mobility Group Box-1 (HMGB1).
Statistical Analysis
The study conducted statistical analyses on recorded measurements of AIF, GFAP, MMP-9, and HMGB-1 levels in both control and treatment groups. The t-test and One-Way Analysis of Variance (ANOVA) were used to assess overall differences among groups, followed by the Tukey Honestly Significant Difference (HSD) test to identify specific groups with significant parameter differences. Data distribution was assessed with the Kolmogorov-Smirnov normality test. In cases where data did not follow a normal distribution, the t-test and a non-parametric alternative to ANOVA were considered.
Results
The response to TBI results in changes in the proteomic transcription profile of microglial cells, which directly affects the morphology and secretion of cytokines and chemokines related to neuronal cell viability. This study investigates MMP9 expression in microglial cells within the brain tissue of rats subjected to TBI. Cellular infiltration and glial activation occur in the injured brain, with neutrophil accumulation mainly occurring within the first 24 hours of injury. Clinical studies have found a higher number of neutrophils after TBI (Figure 1). Experimentally, a higher level in neutrophil accumulation is associated with a higher level of lesion volume and changes in the brain cytokine profile. However, there is no reduction in BBB dysfunction and motor impairment. Immunohistochemistry, employing specific MMP9 antibodies, revealed brown coloration in the cell cytoplasm as an indicator of MMP9 expression. Furthermore, the study explored the impact of MMP9 on neuronal cell apoptosis by assessing AIF expression and DNA fragmentation as a marker of cell apoptosis. AIF expression was detected using specific antibodies, manifested as brown coloration in the neuronal cell cytoplasm, while apoptosis was identified through the TUNEL assay, where brown coloration was observed in the cell nucleus. Photomicrograph of TUNEL assay at 24 hours post-injury are shown in Figure 2, while representation of TUNEL assay with immunoperoxidase staining technique are shown in Figure 3. It can be seen that there is a difference in the distribution of neuron cells undergoing apoptosis, with a significant higher level in the TBI group. The comparative results of protein and enzyme expression between the control group and the TBI group at 24 hours are presented in Table 1. The mean histogram and analysis of GFAP, MMP9, HMGB1, AIF, and apoptosis expression in the brain tissue of rats with the TBI model at 24 hours post-injury are shown in Figure 4. Similarly, the comparative results of protein and enzyme expression between the control group and the TBI group at 72 hours are shown in Table 2, with the mean histogram and analysis of GFAP, MMP9, HMGB1, AIF, and apoptosis expression results at 72 hours also depicted in Figure 5.
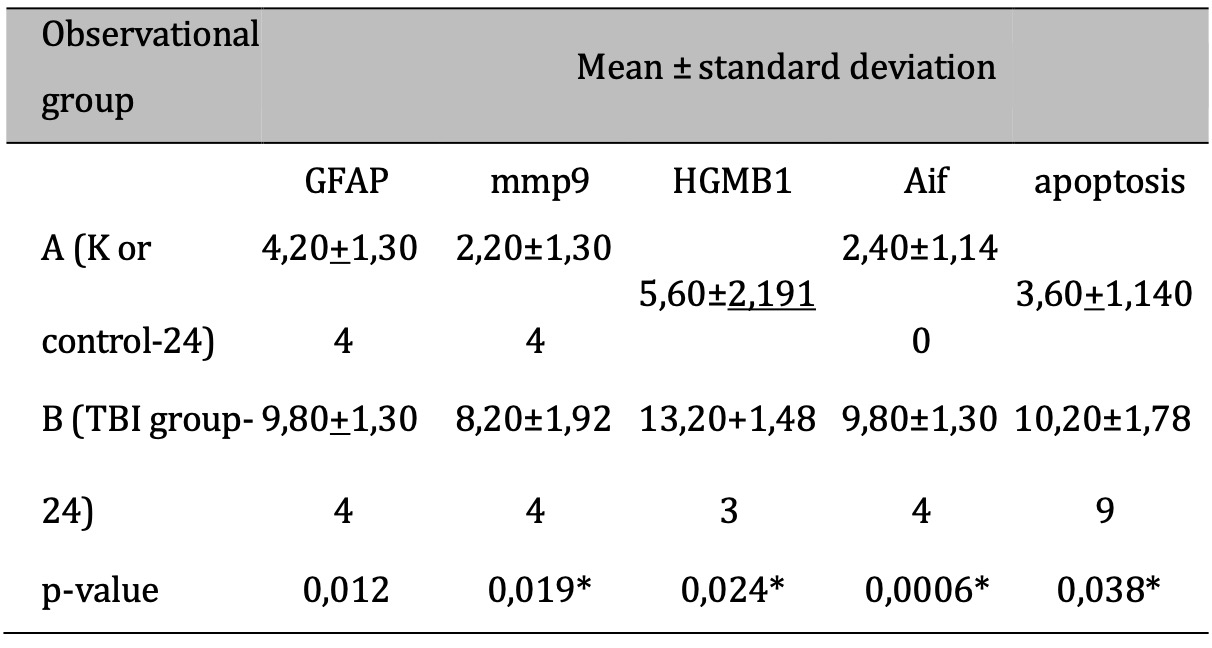
Table 1: The results of the comparative test : Difference in protein & Enzyme expressions of normal rats vs TBI-induced rats (at 24 hours). Note: The results of the T-test are shown in the column of mean±SD. If different letters are shown, it means there is a significant difference (p-value<0.05*). If the same letters are shown, it means there is no significant difference (p- value>0.05)
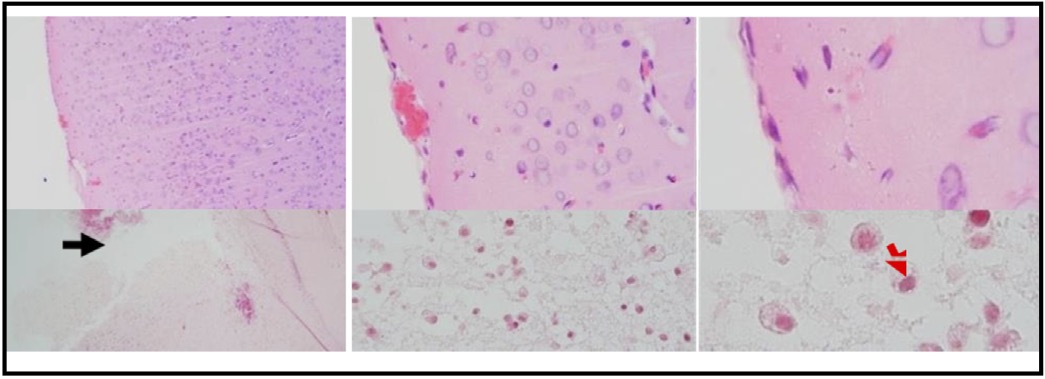
Fig. 1: Photomicrograph of HE staining results, brain tissue of TBI rat model. (A) Normal brain tissue. (B) Brain tissue post-TBI exposure. The images are taken at different magnifications: 100x, 400x, and 1000x. The black arrow indicates the impact injury area in the TBI process. The red arrow indicates the presence of neutrophil cells in the impact area.
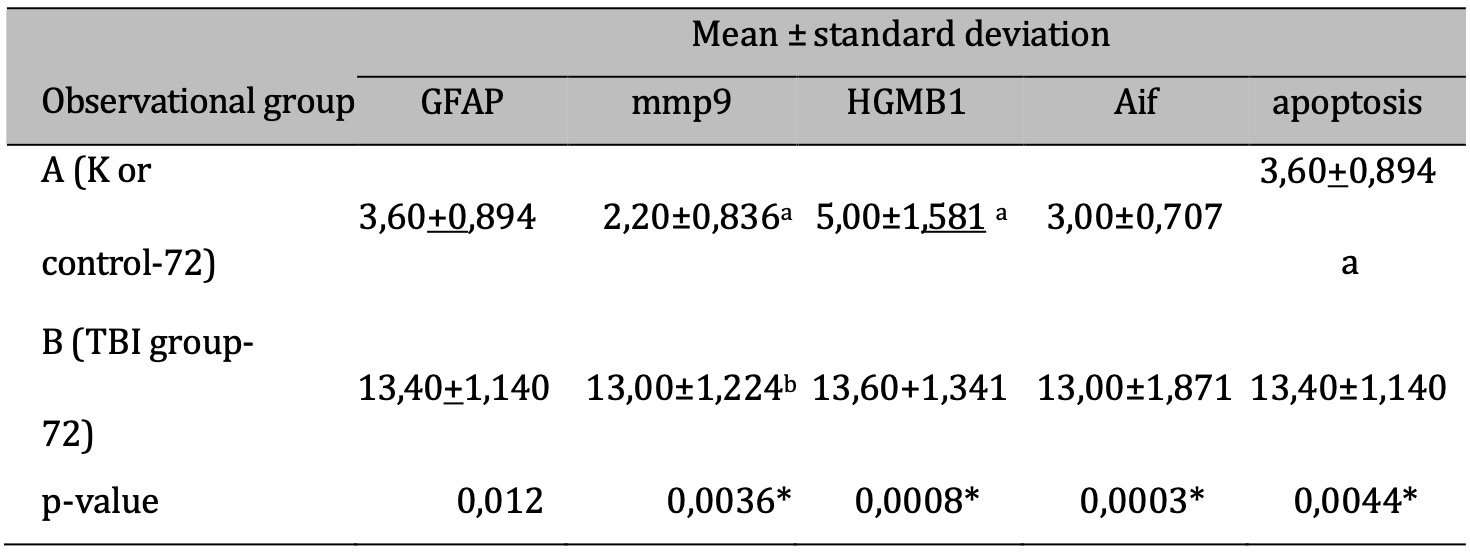
Table 2: The results of the comparative test : Difference in protein & Enzyme expressions of normal rats vs TBI-induced rats (at 72 hours). Note: T-test results are shown in the mean ± SD column. If different letters are present, it means there is a significant difference (p-value <0.05*). If the same letters are present, it means there is no significant difference (p-value> 0.05)
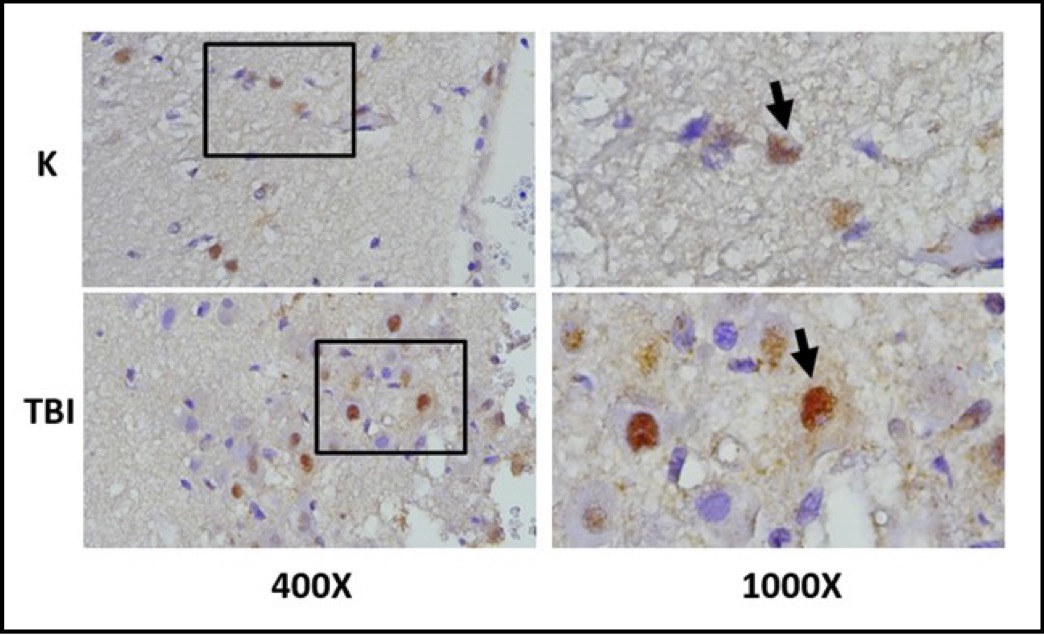
Fig. 2: shows the photomicrograph of TUNEL ASSAY staining results in the brain tissue of TBI mouse model (Picture B). The black box indicates the observed area, and the arrow indicates the neuron cells undergoing apoptosis. It can be seen that there is a difference in the distribution of neuron cells undergoing apoptosis, with a significant higer level in the TBI group.
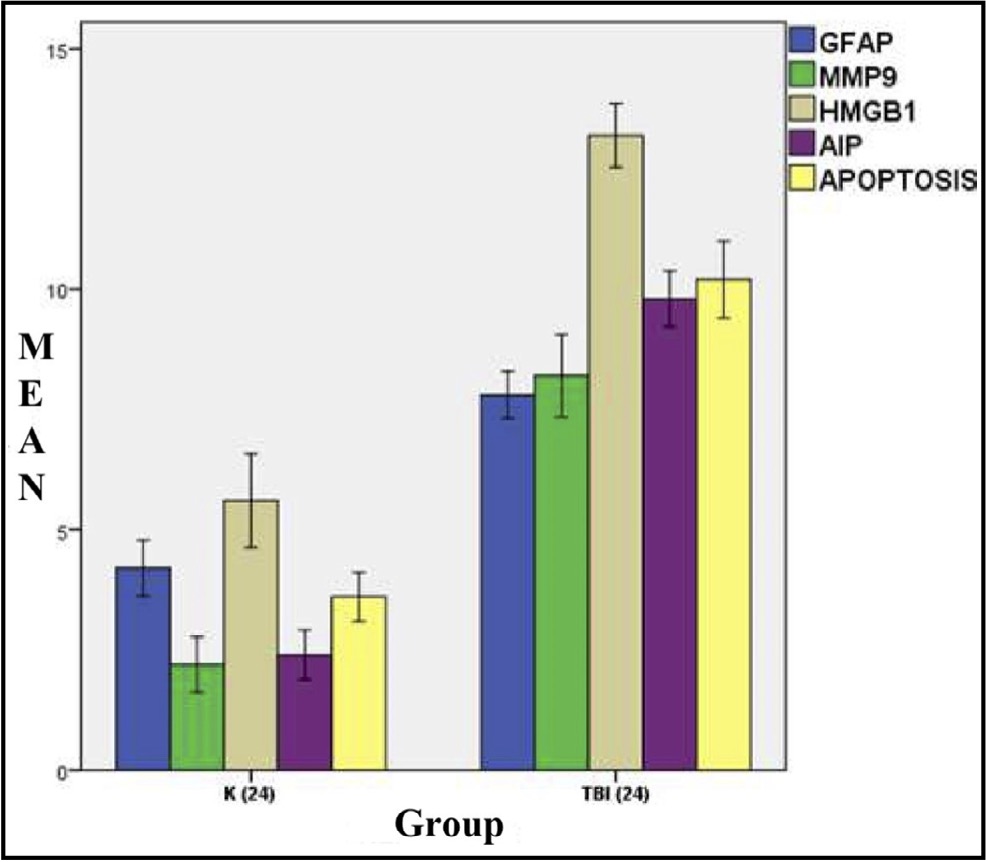
Fig. 3: Mean histogram and analysis of the GFAP, MMP9, HMGB1, AIF, and Apoptosis expression results in the brain tissue of rats with TBI model at 24 hours post-injury.K: Control group (24 hours after injury), referred to as Group A, and Traumatic Brain Injury group (24 hours after injury), referred to as Group B, exhibit a significant difference.
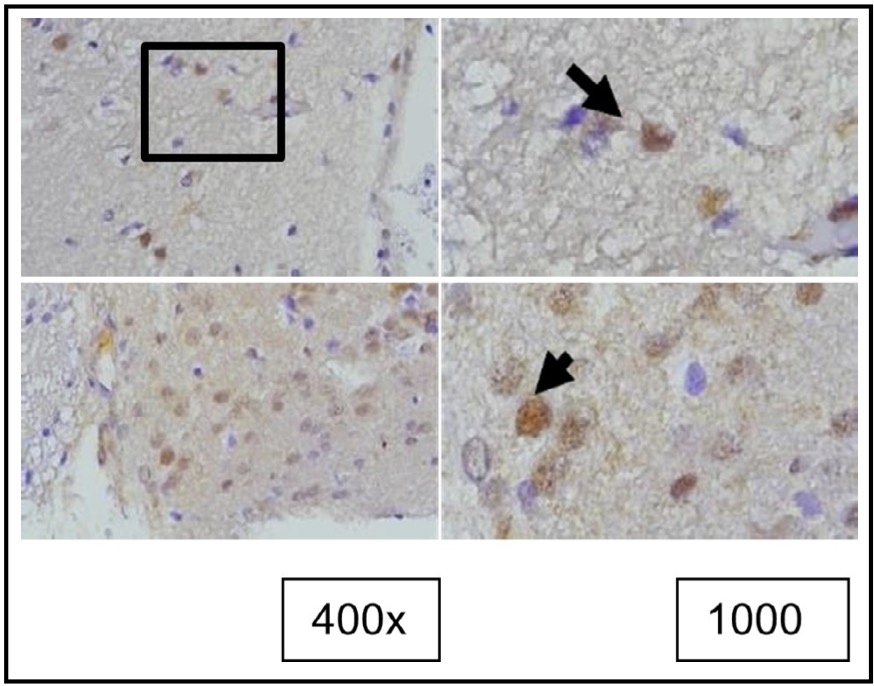
Fig. 4: Representation of the results of tunel staining in rat brain tissue treated with TBI using the immunoperoxidase technique with tunel assay, photo*micrographs at 400x and 1000×. Neurons undergoing apoptosis are marked by brown color in the nucleus (arrow).
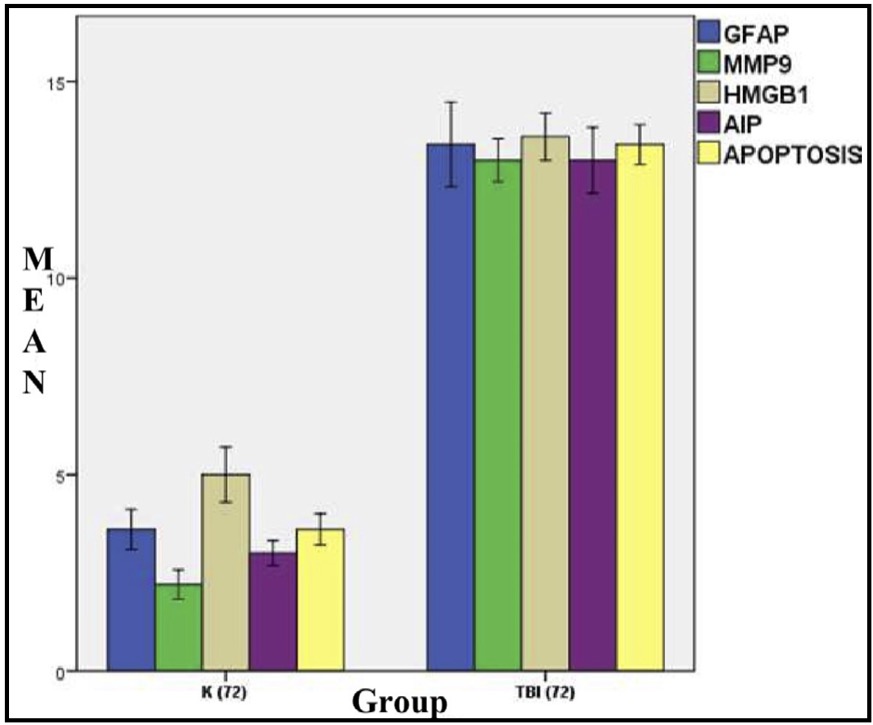
Fig. 5: Mean histogram and analysis of GFAP, MMP9, HMGB1, AIF, and apoptosis expression results in rat brain tissue with TBI model at 72 hours. K: control group (72 hours post-injury); or group A TBI: TBI group (72 hours post-injury) or group.
The response to TBI results in changes in the proteomic transcription profile of microglial cells. This directly affects the morphology and secretion of cytokines and chemokines related to neuronal cell viability, such as the expression of MMP9 and AIF. Additionally, the expression of GFAP and HMGB1 are also observed.
The expression of MMP9 in the brain tissue of rats induced with TBI was observed in microglial cells using immunohistochemistry with anti MMP9 antibodies, marked by brown color in the cell cytoplasm. MMP9 expression are shown in Figure 6. The study also observed its effect on neuronal cell apoptosis through the expression of AIF and the fragmentation of DNA (point of cell apoptosis), with AIF expression examined using immunohistochemistry with specific antibodies and apoptosis detected using the TUNEL assay to observe DNA fragmentation. AIF expression was observed with brown color in the neuronal cell cytoplasm, while apoptosis was observed with brown color in the cell nucleus. AIF expression is shown in Figure 7.
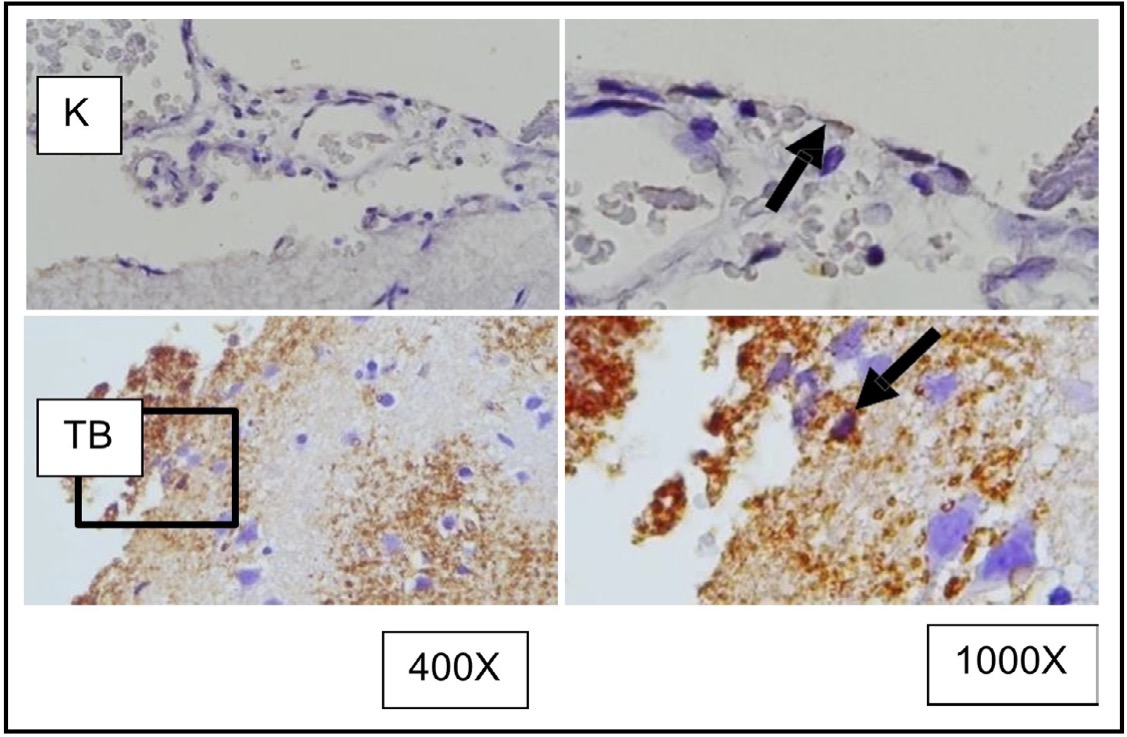
Fig. 6: Representation of immunohistochemical staining results of rat brain tissue with TBI treatment using immunoperoxidase technique with anti-GFAP antibody, photomicrographs at 400x and 1000x magnification. Microglial cells with GFAP expression are marked with brown color in the cytoplasm (arrow). (K represents the control group, while TBI represents the group receiving TBI.
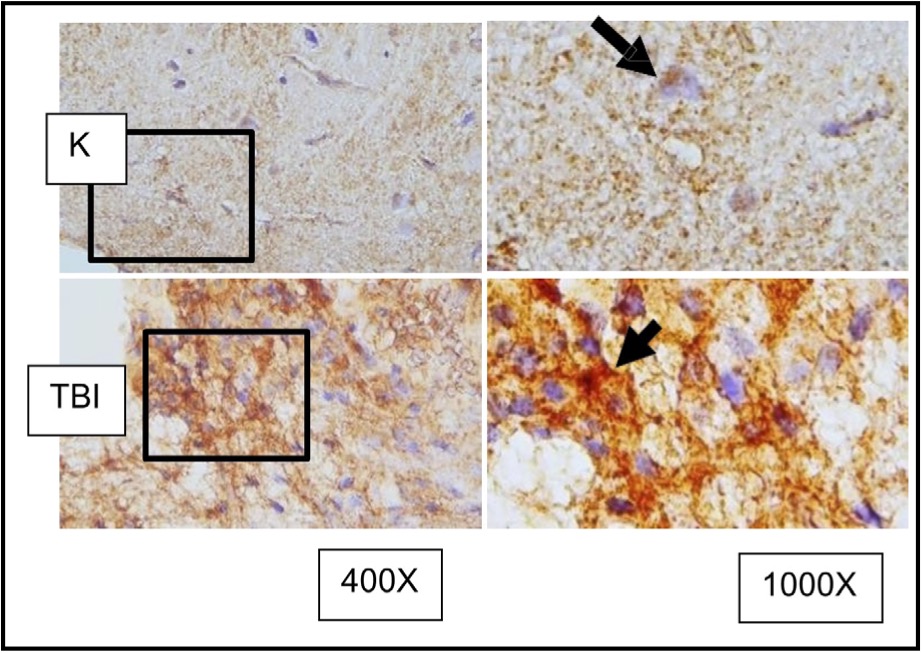
Fig. 7: Representation of immunohistochemical staining results from brain tissue samples of TBI-induced rats using anti-MMP9, with photomicrographs at 400x and 1000× magnification. Neurons with MMP9 expression are marked by brown color in the cytoplasm of cells (arrow).( K control; TBI rats with TBI treatment.
The presence and levels of GFAP are often used as markers for astrocyte activation and gliosis, which is a reactive response of glial cells to brain injuries. The GFAP expression in brain tissues is examined using the anti-GFAP antibodies (Figure 8).
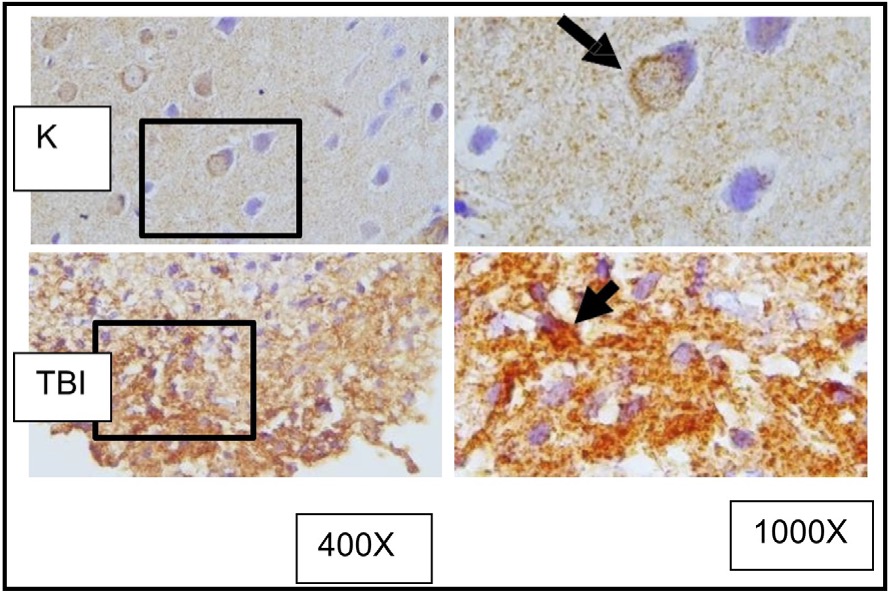
Fig. 8: shows the representation of immunostaining results from rat brain tissue sections with TBI treatment using immunoperoxidase technique using anti- HMGB1 antibody, photomicrographs at 400x and 1000x. Neurons with HMGB1 expression are marked with brown color in the cytoplasm of the cell (arrow). K indicates the control group, TBI rats with TBI treatment. It is known that HMGB1 is normally localized in the cell nucleus (arrow in group K) and becomes inflammatory when translocated to the cytoplasm or extracellular space. ( K control; TBI rats with TBI.).
HMGB1 is a protein that plays a crucial role in the response to TBI. In the context of TBI, HMGB1 is released from damaged cells and serves as a pro-inflammatory mediator, contributing to the inflammatory response following the injury. The HMGB1 expression in brain tissues is examined using the anti-HMGB1 antibodies (Figure 9). It is known that HMGB1 is normally localized in the cell nucleus (arrow in group K) and becomes inflammatory when translocated to the cytoplasm or extracellular space.
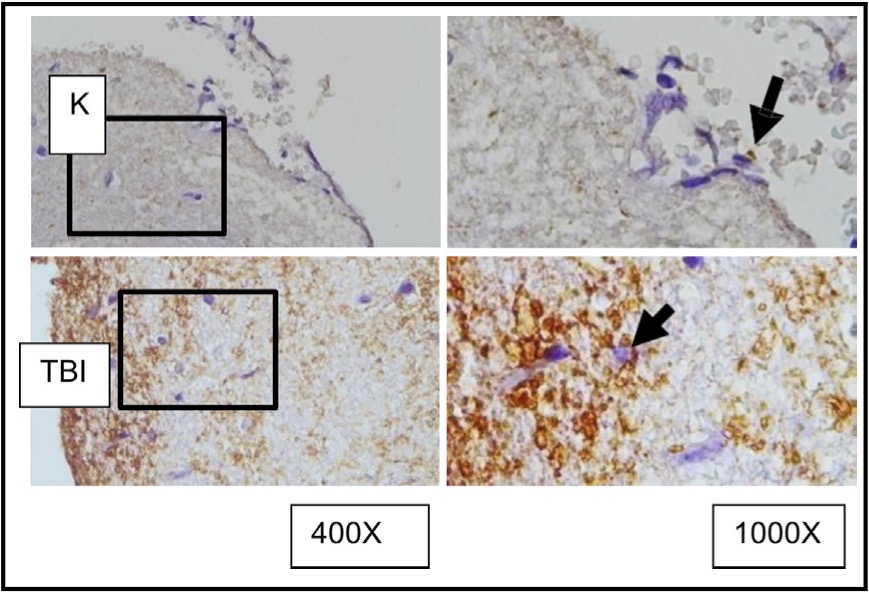
Fig. 9: Representation of immunohistochemical staining results of brain tissue samples from TBI-treated rats using immunoperoxidase technique with anti- AIF, photomicrographs at 400x and 1000x magnification. Neurons with AIF expression are marked in brown in the cytoplasm (arrow). K control TBI rats with TBI.
This study observed the role of OLE in the expression of GFAP, HMGB1, and AIF in microglia of rat brain tissue due to TBI induction and its association with the occurrence of apoptosis in surrounding neuron cells. Secretion of cytokines and chemokines related to neuronal cell viability are observed after OLE treatment using immunohistochemistry assessments. The comparison of GFAP expression between groups treated with TBI and OLE are shown in Figure 10, GFAP with and without OLE in Figure 11, HMGB1 in Figure 12, and AIF in Figure 13. The results of TUNEL assay kit with immunoperoxidase staining on groups treated with TBI and OLE is shown in Figure 14.
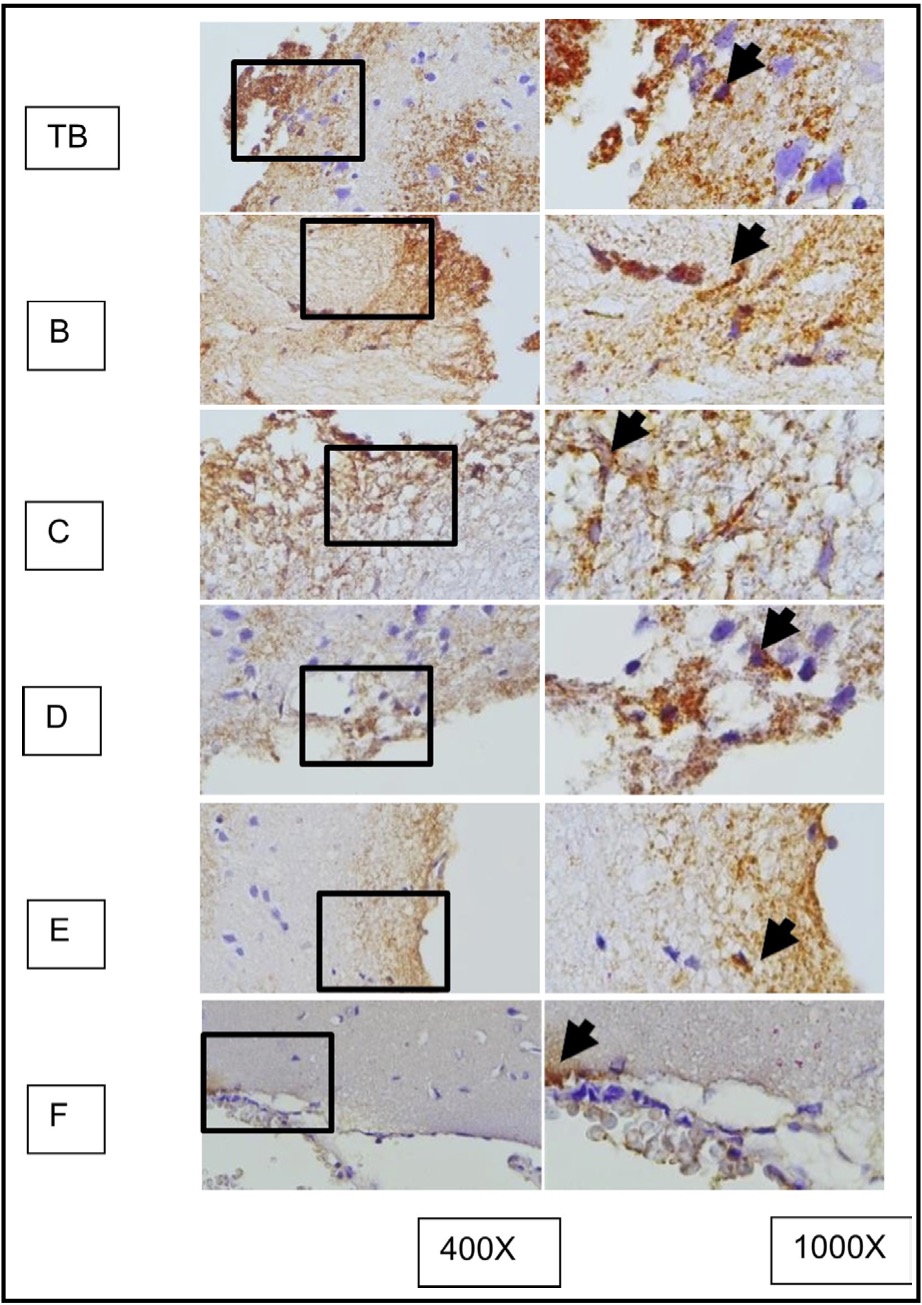
Fig. 10: represents the results of immunohistochemical staining of mouse brain tissue samples treated with TBI and OLE treatment, using the immunoperoxidase technique with anti-gfap, photographed at 400x and 1000x magnifications. Glial cells expressing GFAP are marked with brown color in the cytoplasm (arrow). A represents TBI group; B, C, D, E, and F represent different doses of OLE treatment group.
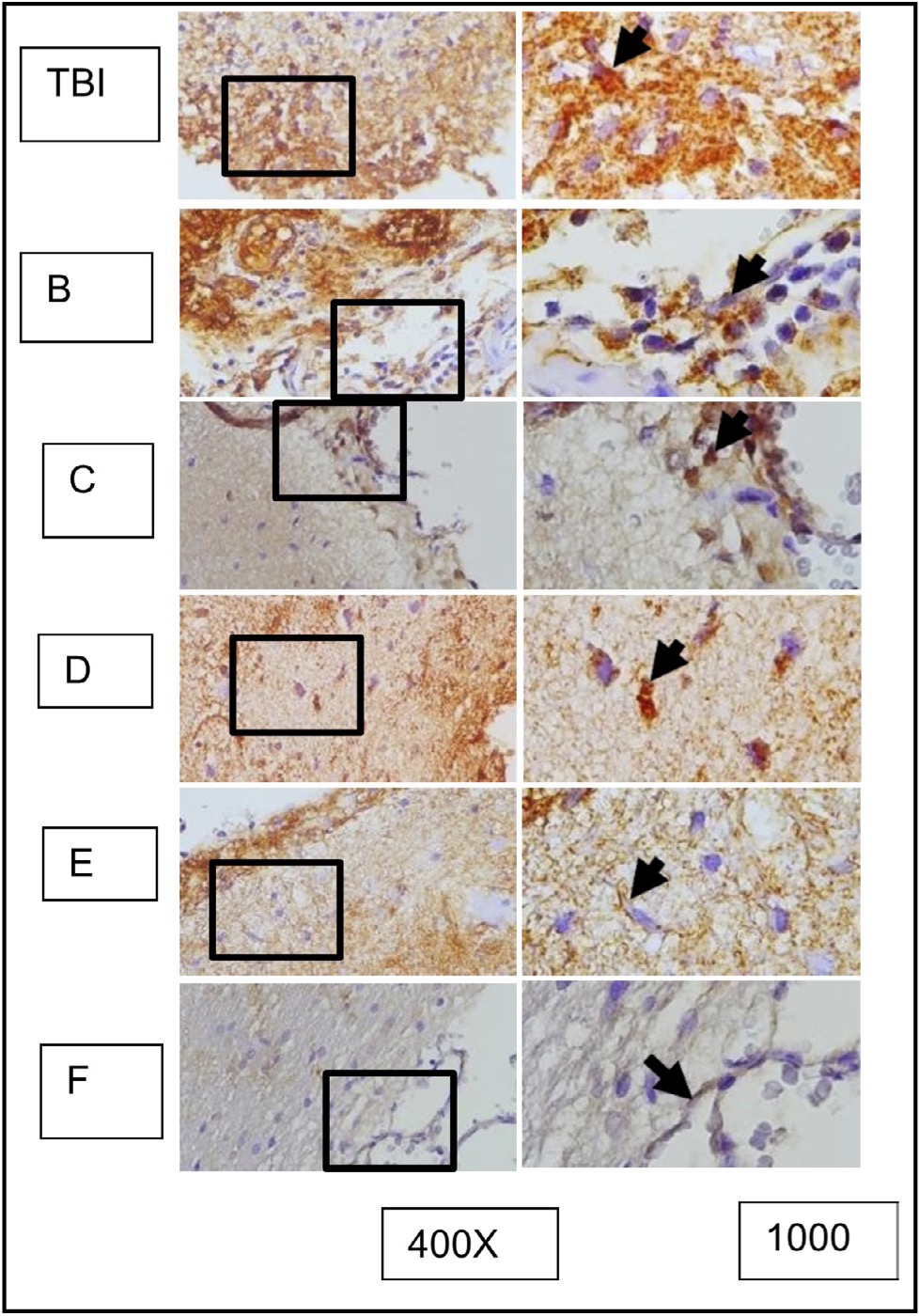
Fig. 11: Shows the representation of immunocytochemistry staining results of brain tissue sections from rats subjected to TBI with and without OLE treatment, using immunoperoxidase technique with anti-gfap, photomicrographs at 400x and 1000x. Glia cells expressing GFAP are marked with brown color in the cell cytoplasm (arrow). Group A represents the TBI rats, and groups B, C, D, E, and F represent the OLE treatment groups with various doses.
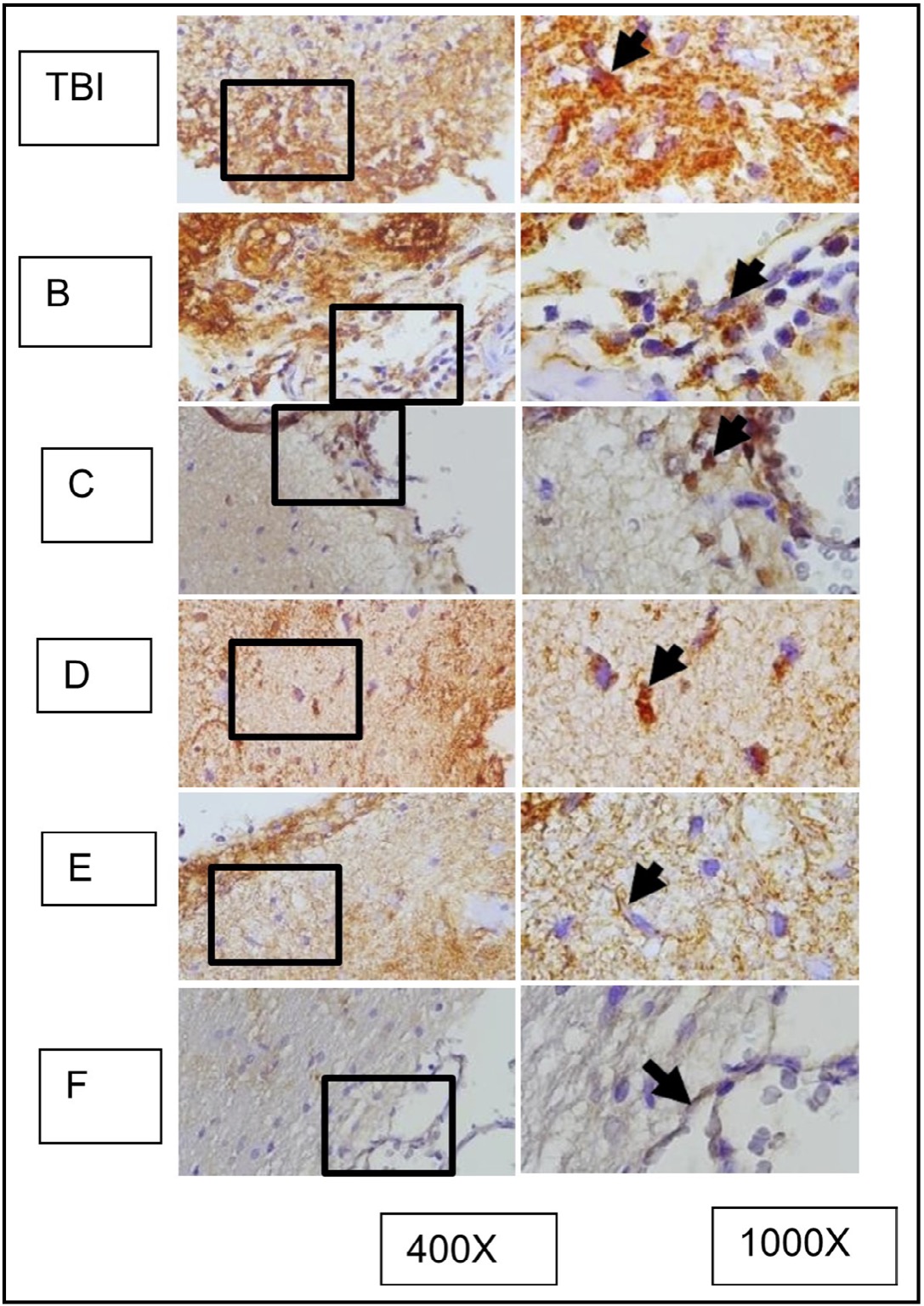
Fig. 12: represents the results of immunohistochemical staining of brain tissue sections from TBI-treated rats, stained with anti-HMGB1 antibodies and visualized with immunoperoxidase technique at 400x and 1000× magnification. Neurons expressing HMGB1 are marked with brown color in the cytoplasm of the cell (arrow). TBI-treated rats without treatment are marked as group TBI; B, C, D, E, and F represent different doses of OLE treatment. It is known that HMGB1 is normally localized in the nucleus (arrow in group K) and becomes inflammatory when translocated to the cytoplasm or extracellular space.
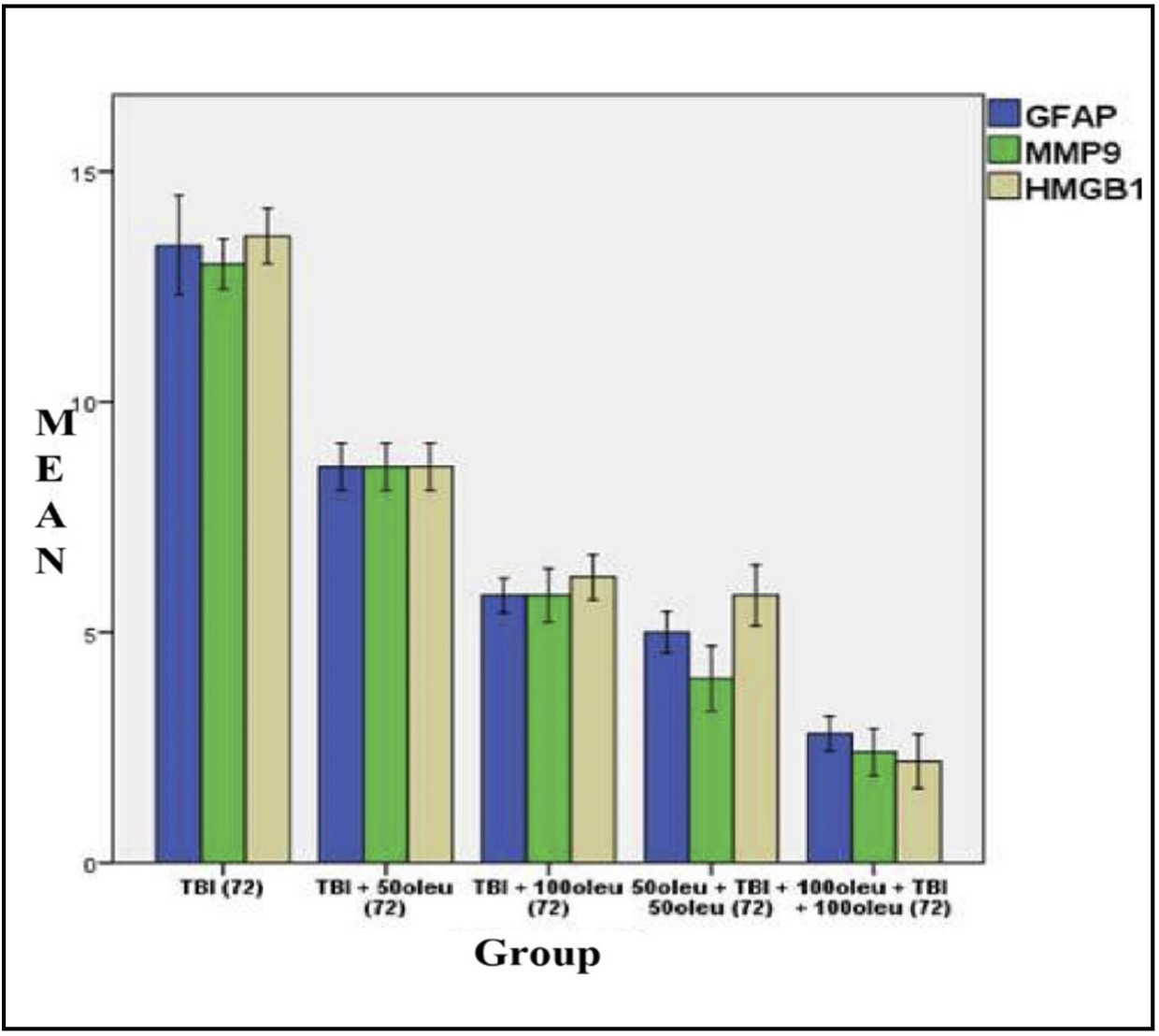
Fig. 13: shows the mean/average histogram and analysis results of GFAP, MMP9, and HMGB1 expression in mouse brain tissue with TBI model with OLE administration at 24 hours post-treatment (differences compared to control group)
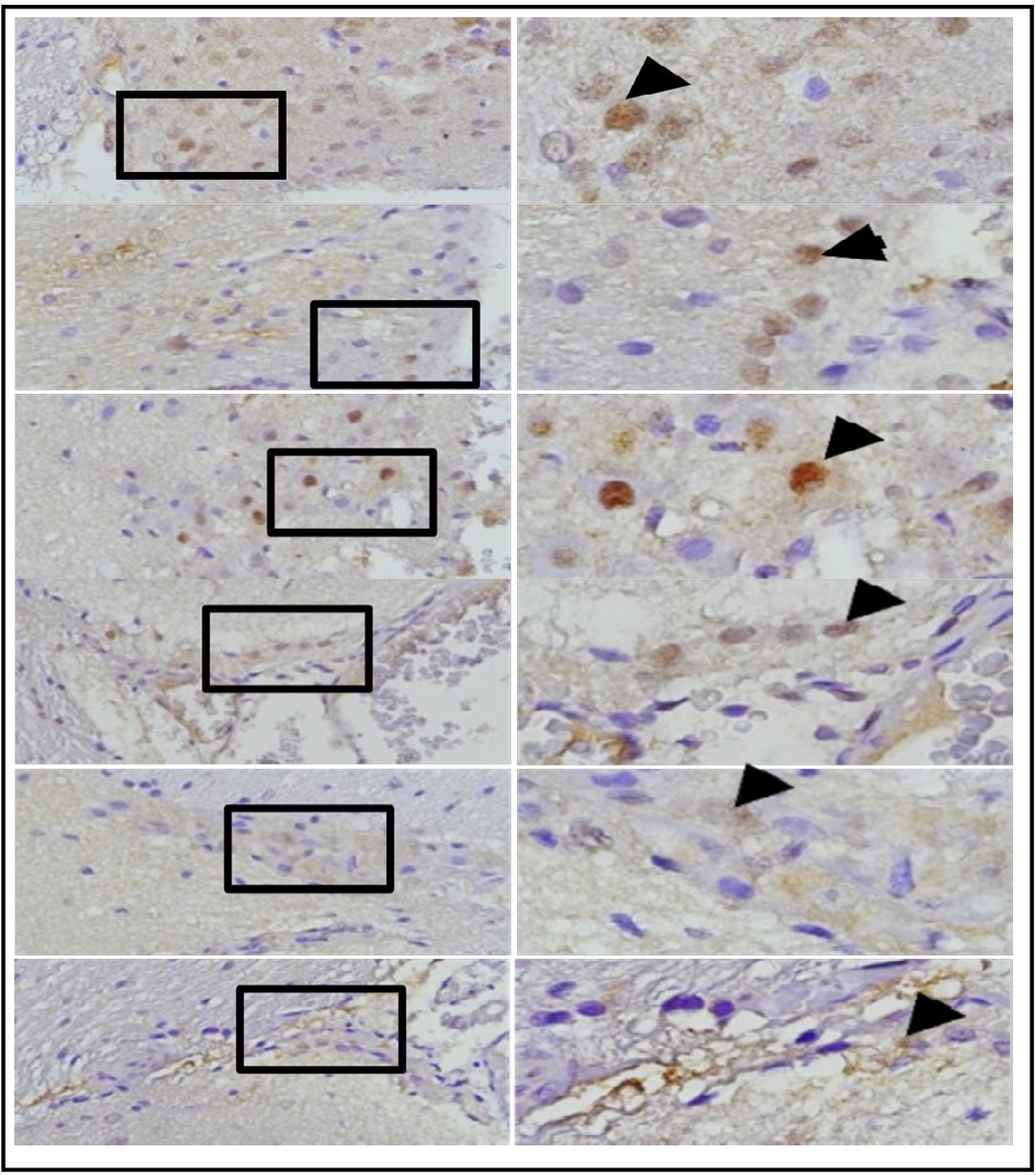
Fig. 14: Mean histogram and analysis results of GFAP, MMP9, and HMGB1 expression in the brain tissue of TBI-
The comparative results of protein and enzyme expression between TBI model and OLE administration with various doses at 24 hours post-treatment compared to control group at 24 hours are presented in Table 3. Mean histogram analyses of GFAP, MMP9, and HMGB1 expression in brain tissue with TBI model and OLE administration with various doses at 24 hours post-treatment compared to control group is shown in Figure 15. Similarly, the comparative results of protein and enzyme expression between groups with different OLE doses at 72 hours are shown in Table 4, with the mean histogram and analysis of GFAP, MMP9, HMGB1 expression results at 72 hours also depicted in Figure 16. The results showed that there are significantly lower levels of MMP9 expression in the group with TBI with OLE administration compared to the TBI treatment group (p < 0.05)
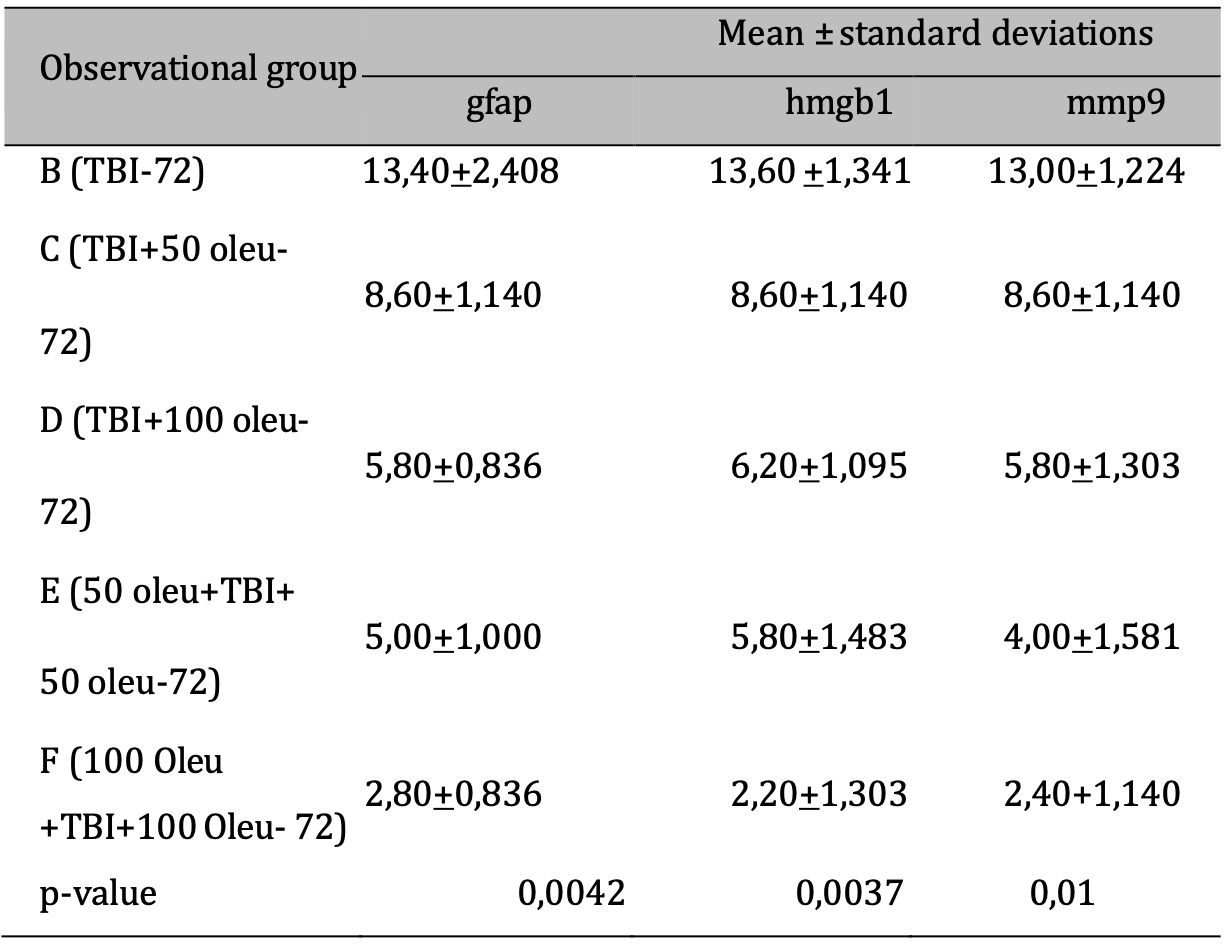
Table 4: The results of the comparative test: Protein & Enzyme Expression under Different Oleuropein Doses compared to control (at 72 hours). Note: The results of the ANOVA test are shown in the column of mean±SD, if the letters are different, there is a significant difference (p-value 0.05*), and if the letters are the same, there is no significant difference (p-value > 0.05)
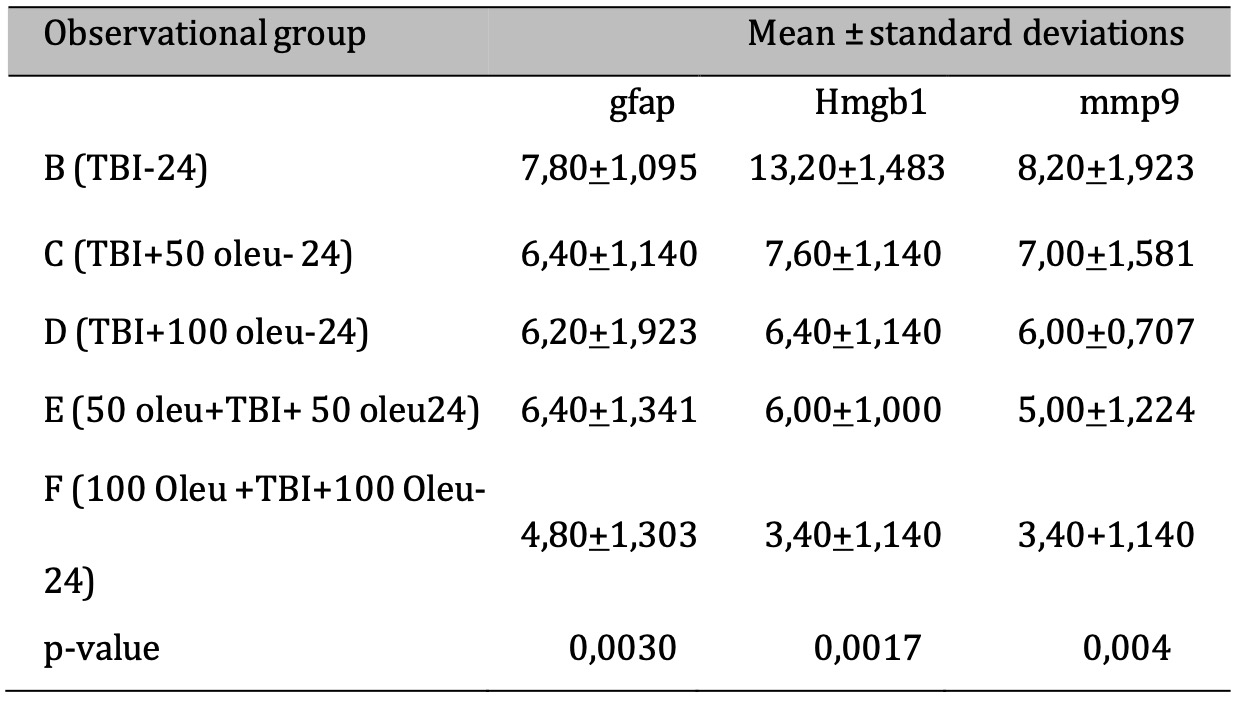
Table 3: The results of the comparative test: Protein & Enzyme Expression under Different Oleuropein Doses compared to control (at 24 hours ). Note: The ANOVA test results are presented in the column as mean±SD. If they contain different letters, it indicates a significant difference (p-value < 0.05), and if they contain the same letters, it indicates no significant difference (p-value >
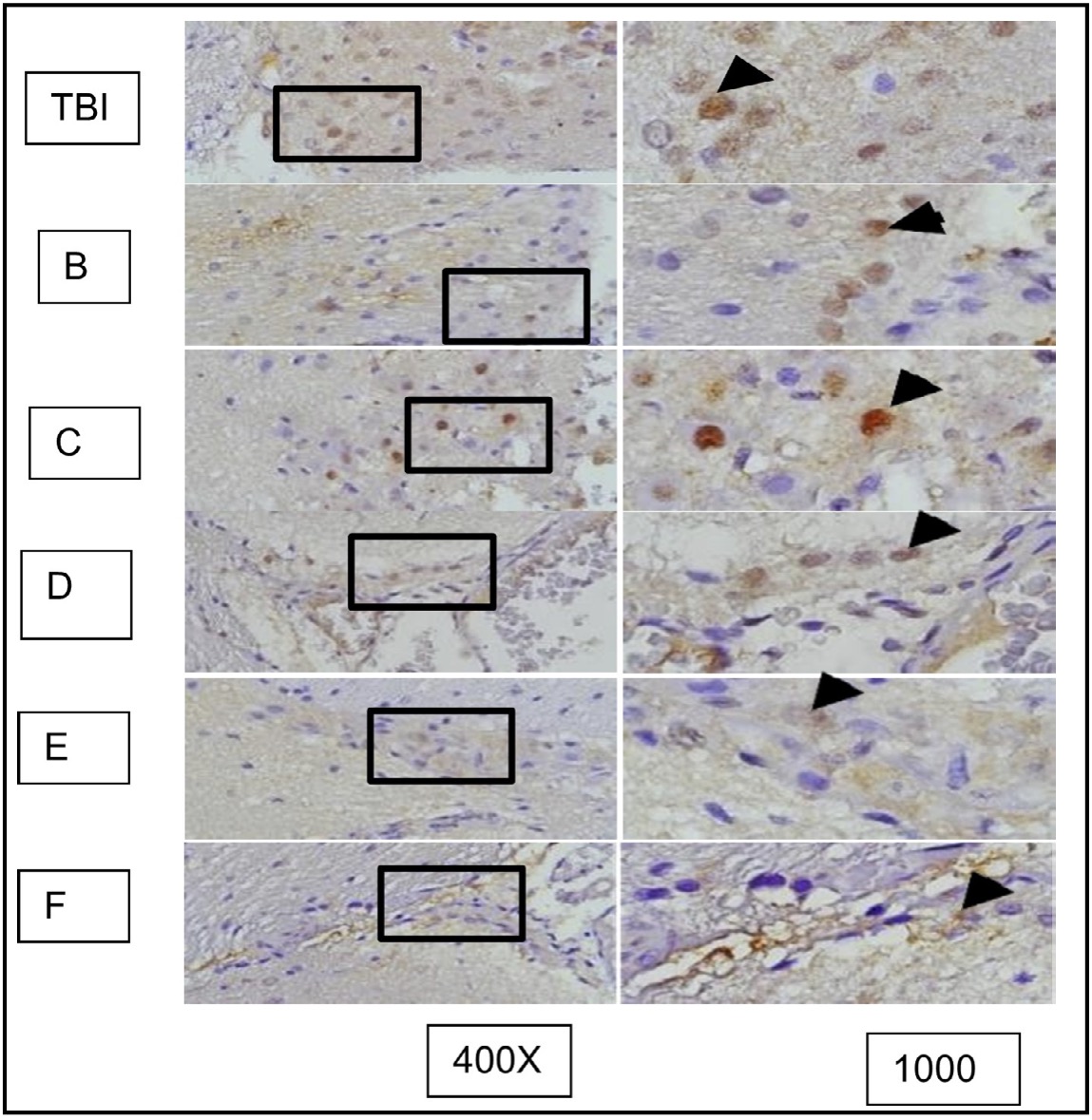
Fig. 15: represents the results of TUNEL staining on mouse brain tissue samples with TBI and OLE treatment, stained with immunoperoxidase technique usingTUNEL assay kit, photomicrographs at 400x and 1000x. Neurons undergoing apoptosis are marked with brown color in the nucleus of the cell (arrow). TBI group represents ratswith TBI treatment; B, C, D, E, and F represent the OLE treatment groups with various doses.
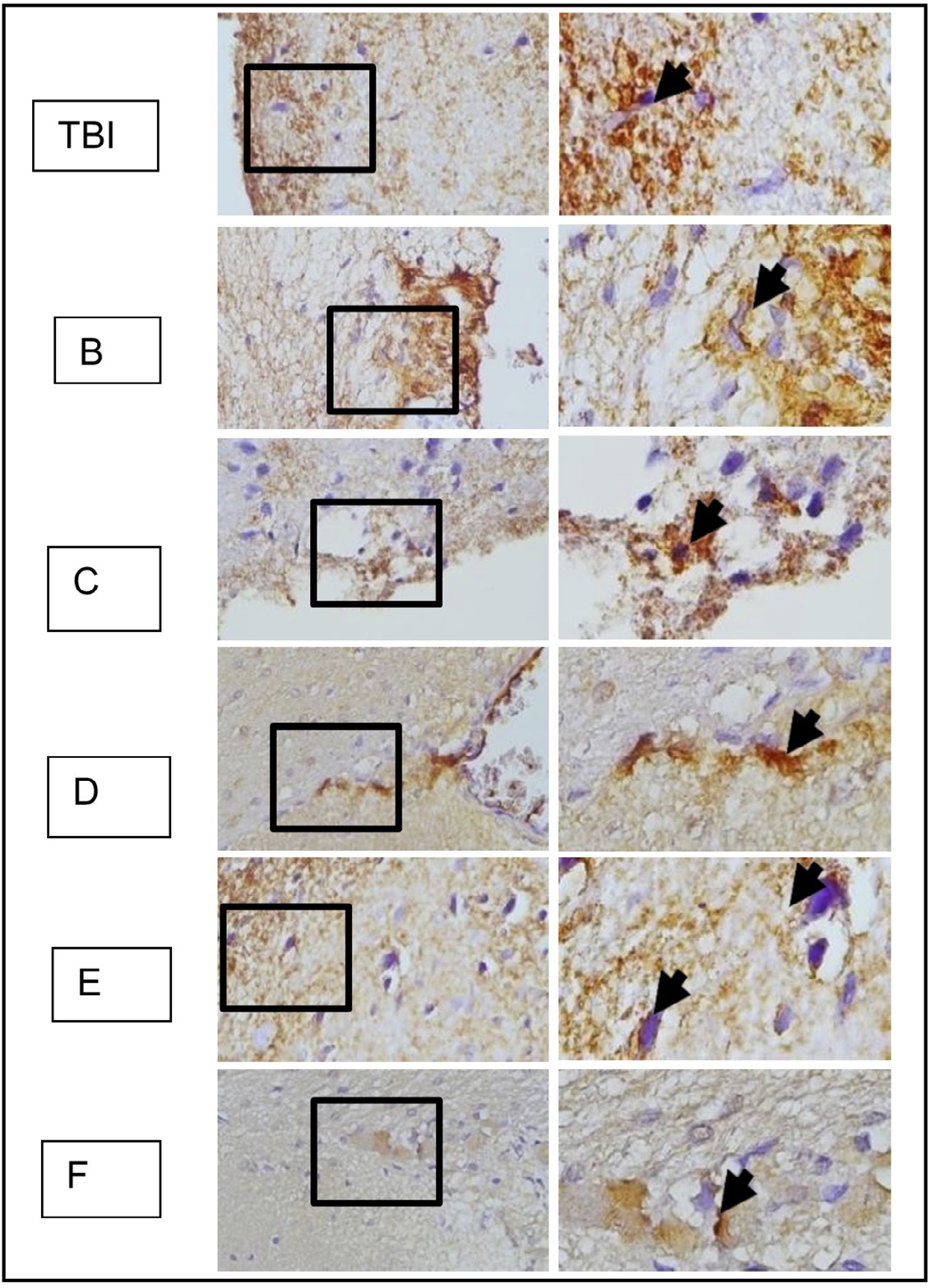
Fig. 16: represents the results of immunohistochemical staining of brain tissue samples from rats with TBI treatment and OLE treatment, using anti-AIF antibodies and the immunoperoxidase technique, photographed at 400x and 1000x magnification. Neurons expressing AIF are marked with brown color in the cytoplasm (arrow). TBI group with TBI treatment; B, C, D, E, and F groups with OLE treatment at various doses.
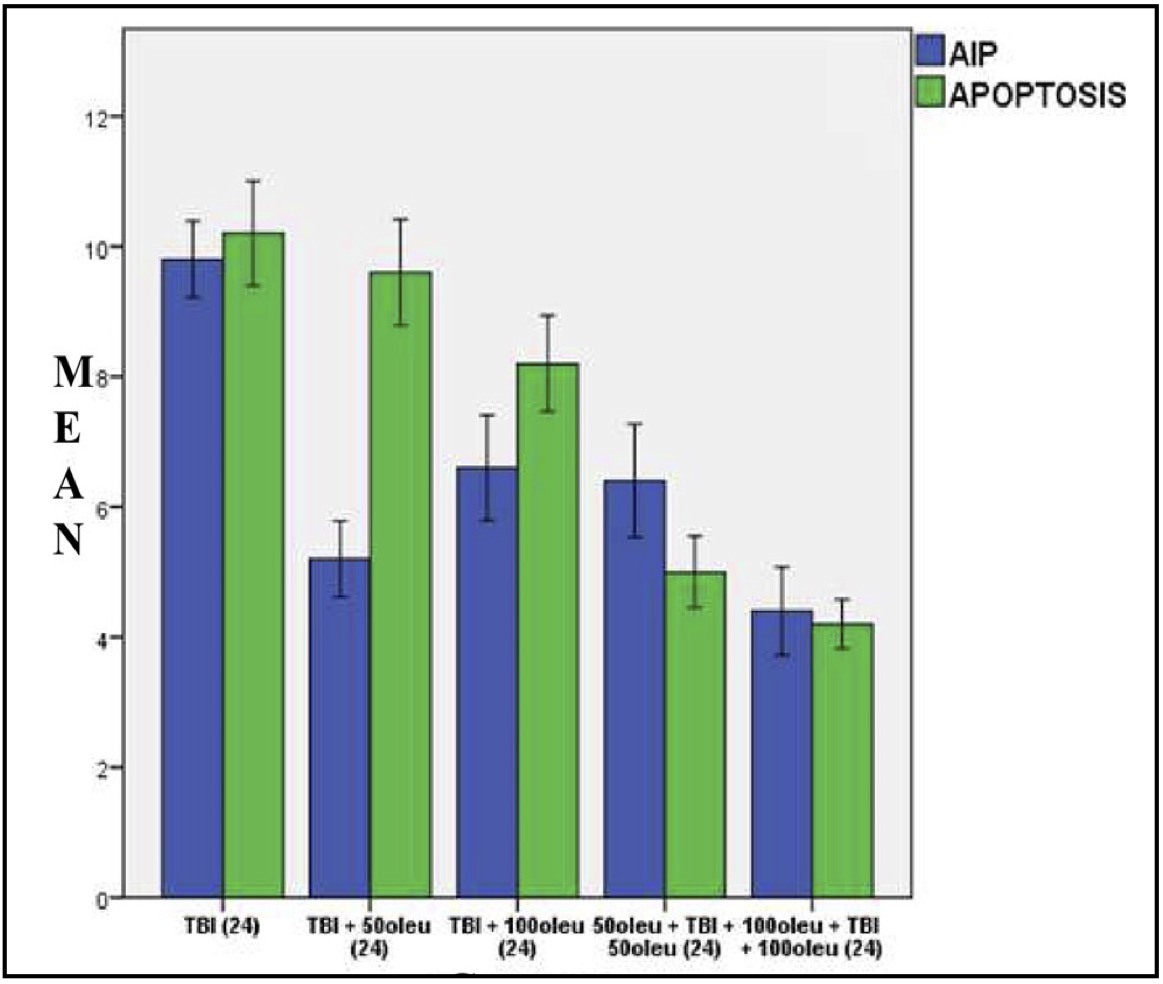
Fig. 17: Mean histogram and analysis results of AIF expression and apoptosis incidence in the mouse brain tissue with TBI model and OLE administration at 24 hours post-treatment.
OLE’s role in reducing apoptosis was investigated in neurons using immunohistochemical techniques to observe AIF and a TUNEL assay for apoptosis. The comparison of apoptosis incidence and AIF expression in brain tissue with TBI model and OLE administration in various doses at 24 hours post-treatment is shown in Table 5 and mean histogram analysis in Figure 17. Analyses at 72 hours post treatment are shown in Table 6 and mean histogram analysis in Figure 18. The results showed a lower level in the number of apoptotic neurons in the TBI group with OLE treatment compared to the TBI treatment group (p <0.05).
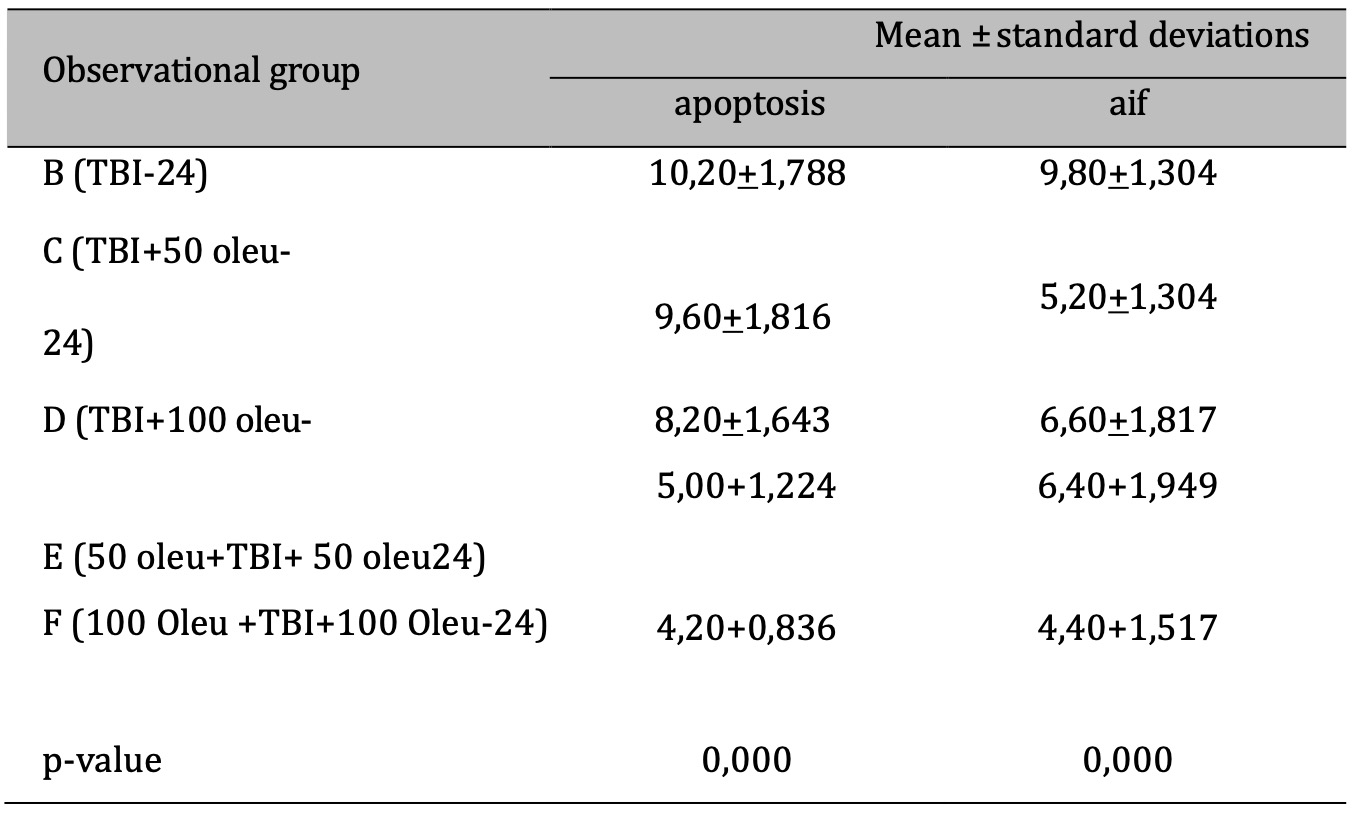
Table 5: The results of the comparative test: AIF vs apoptosis under Different Oleuropein Doses compared to control (at 24hours). Note: The results of the Anova test are shown in the column of mean±SD. If the letters are different, there is a significant difference (p-value 0.05), and if the letters are the same, there is no significant difference (p-value > 0.05)
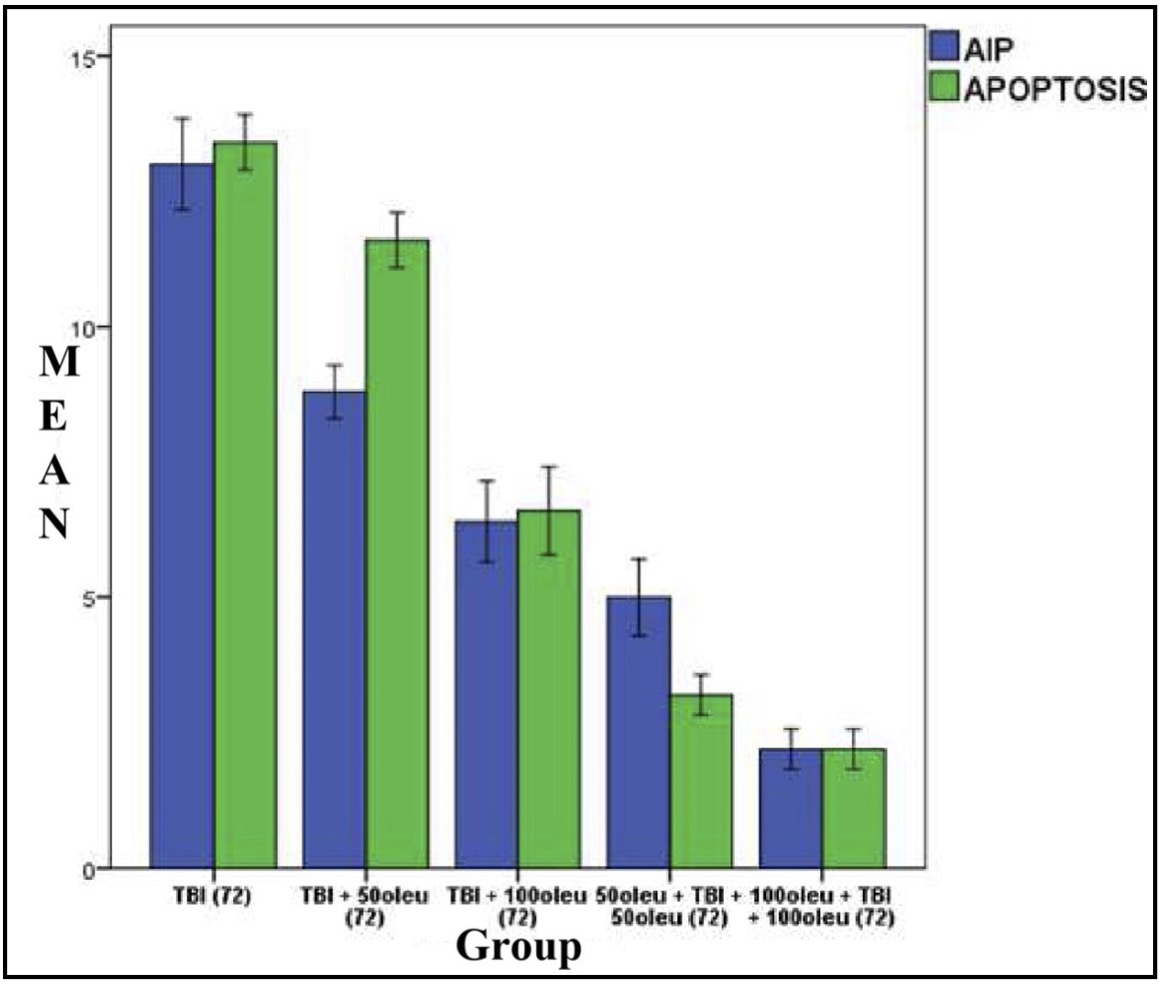
Fig. 18: Mean histogram and analysis results of AIF expression and apoptosis incidence in mouse brain tissue with TBI model treated with OLE at 72 hours post- treatment
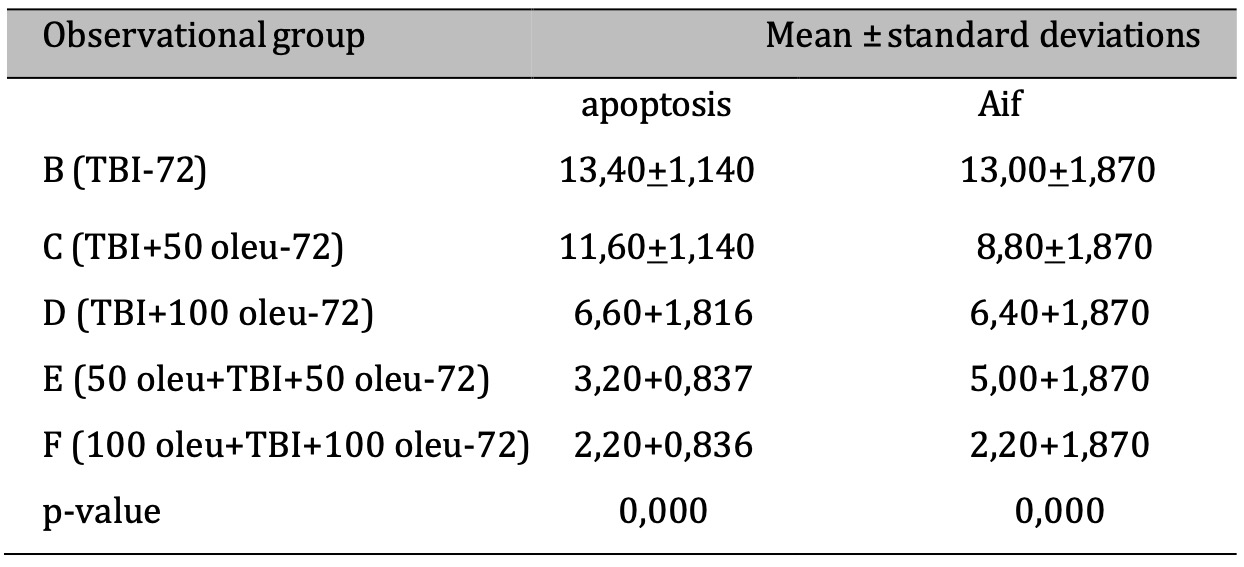
Table 6: The results of the comparative test: AIF vs apoptosis under Different Oleuropein Doses compared to control (at 72hours). Note: The ANOVA test results are shown in the column of mean ± SD, if the. letters are different, there is a significant difference (p-value 0.05), and if the. letters are the same, there is no significant difference (p-value > 0.05)
Discussion
Induction of Apoptosis through AIF Pathway on MMP-9 Levels in Traumatic Brain Injury Rat Model.
The term “apoptosis” refers to a type of cell death that is regulated by themitochondria and is characterised by internucleosomal DNA fragmentation,which can be seen in real time using a technique called TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling). Following traumatic injury, neuronal apoptosis may serve as a physiological and protective response to damage since it is a method for removing surplus neurons with little immune system activation [20]. However, transgenic rats that overexpress anti-apoptotic proteins exhibit a significantly lower level of damage to the cortex and hippocampi after traumatic brain injury (TBI). Consequently, excessive apoptosis-related pathway activation can be hazardous, particularly under pathological states. Preclinical research has shown that the pericontusional area and the hippocampus both exhibit neuronal cell loss [21, 22].
Apoptotic cells were detected in the wounded cortex as early as 24 hours following the injury using a model of moderate lateral fluid percussion, although they took longer to appear in the hippocampus and thalamus, peaking at 48 hours and 2 weeks following the injury, respectively. Mitochondria trigger various pathways of apoptotic signaling, through interaction among the bcl-2 family of proteins, including cytochrome c, apoptosis-inducing factor (AIF), second mitochondria-derived activator of caspases (Smac), endonuclease G (Endo G), and mitochondrial-derived activator of caspases (Smac), to release pro- apoptotic proteins from the mitochondrial intermembrane space, resulting in apoptosis. This route is called the intrinsic pathway [23]. One of these is the downstream interaction protein of the Bcl-2 family mechanism in the intermembrane space, including cytochrome c, AIF, Smac, and Endo G, released and interacting with one another following TBI. Pro- apoptotic proteins are then released as a result of their interaction. Procaspase- 9 is activated by the interaction of cytochrome c with apoptosis protease- activating factor-1 (apaf-1) to produce the apoptosome. DNA fragmentation and apoptosis result from the degradation of the activated caspase DNase inhibitor by caspase-3 after caspase-9 activates procaspase-3. An enzyme that repairs DNA damage after TBI which is called poly(ADP-ribose) polymerase (PARP), can also be activated by caspase-3. Although PARP is implicated in necrosis and apoptosis, after cerebral ischemia, caspases cleave the 89- and 21-kDa fragments, which are linked to apoptosis. Smac contributes to the activation of caspase. Following TBI, Smac is released from mitochondria and attaches to the X-linked inhibitor of apoptosis protein, neutralising its actions and causing further apoptosis [24-23]. Other studies have shown the significance of the caspase-independent pathway. Following TBI, membrane permeabilization causes the release of a flavoprotein containing NADH oxidase in the mitochondrial intermembrane space; apoptosis-inducing factor (AIF), into the cytosol [25]. AIF then triggers apoptosis in the nucleus after translocating there. Additionally, this apoptotic pathway is independent of caspases, apf-1, and cytochrome c. AIF released from mitochondria and translocation to the nucleus are also regulated by cyclophilin A, HSP-70, adn PARP-1. Following TBI, PARP-1 inhibition has a neuroprotective effect [26, 27]. Cyclophilins belong to peptidylprolyl cis-trans isomerases. Cyclophilin A takes part in the nuclear translocation of AIF from the cytosol to the nucleus and facilitates the chromatinolytic effects of AIF. Through their role as chaperones, heat shock proteins from the HSP70 family protect neurons. By preventing the production of apoptosomes and the nuclear translocation of AIF, HSP70 binding to Apaf-1 and AIF counteract their respective pro-apoptotic actions. When HSP70 is overexpressed, it inhibits caspase-dependent pathways and sequesters AIF, both of which reduce the severity of ischemic brain injury. Contrary to caspase-dependent cell death, AIF-mediated apoptosis can occur in the presence of disturbed bioenergetic status; this can easily be seen in nuclear lesions after cerebral ischemia [26, 28]. In fact, under conditions of energy deficiency, mitochondria are more prone to release AIF, resulting in caspase-independent apoptosis. As a consequence, in cases of severe TBI where bioenergetic disturbance is more likely to occur, AIF-induced apoptosis may be more common. On the other hand, caspase-mediated apoptosis is more prone to happen in moderate TBI, where mitochondrial energy utilisation is less of a problem. In a focal cerebral ischemia animal model, Endo G is also known to translocate to the nucleus and result in fragmentation of DNA, which may potentially apply to TBI injury [29, 30]. The study found elevated levels of apoptotic neurons through the AIF pathway in rats undergoing traumatic brain injury (TBI) treatment, a phenomenon associated with increased MMP-9. The response to TBI results in a change in the proteomic transcription profile of microglia, which directly causes changes in morphology and cytokine and chemokine secretion associated with neuron cell viability. This study examined the expression of MMP-9 in microglia brain tissue of rats induced with TBI. MMP-9 expression was observed in microglial cells using specific antibodies labeled with brown color on the cytoplasm of cells [32]. The study also observed the effect on neuron cell apoptosis through AIF expression and DNA fragmentation (point of cells apoptosis). AIF expression was observed using specific antibodies in immunohistochemistry, and apoptosis was examined using the TUNEL assay to observe DNA fragmentation. AIF expression was observed with brown color in the cytoplasm of neurons undergoing apoptosis, and apoptosis was observed with brown color in the nucleus of cells [33]. The outcomes of our research indicate a significantly elevatedexpression of MMP-9 in the TBI group compared to the normal group (p < 0.05). Furthermore, the observation of neuron cells revealed a markedly higher incidence of apoptosis, as evidenced by AIF expression and DNA fragmentation, in the TBI group compared to the normal group (p < 0.05).
This study found a significant improvement in the group of rats with TBI in the first 24 hours compared to the control group during the same observation period, as well as at 72 hours after TBI induction. MMP9 and AIF (in this study), continued to have elevated level in the next 24 to 72 hours of TBI treatment. Previous studies have suggested that MMPs (matrix metalloproteinases), which are involved in cell death, tissue repair, and morphogenesis, may be used as a therapeutic target for acute brain injury. This study looked at the MMP-2 and MMP-9 expressions after traumatic brain injury in rat pups. The researchers found that MMP-2 and -9 levels increased after injury and that an MMP inhibitor reduced brain damage. However, the exact mechanisms behind MMP-mediated brain damage are still unknown. It is thought that MMPs may digest matrix proteins, including laminin, which is widely distributed in the brain parenchyma and can affect cell-matrix interactions and cell survival. Other enzymes, such as plasmin, may also degrade laminin after brain injury. In addition, previous research has shown that MMP-9 can be activated by S-nitrosylation and contribute to neuronal apoptosis during cerebral ischemia [34-38]. The Effect of Oleuropein Administration and Administration Duration on HMGB1 Expression of Microglia Cells in the Brain Tissue of Traumatic Brain Injury Rat Model. This study examined the role of OLE in the expression of MMP9 in microglia in the brain tissue of rats induced with TBI and its association with the occurrence of neuronal cell apoptosis around it. As before, the method used was immunohistochemistry. These findings demonstrate that the expression of microglia was significantly lower in the TBI group treated with OLE at 24 hours compared to the TBI treatment group (p < 0.05). Consistent with this, it appeared that the expression of MMP9 also were significantly lower in the group with TBI-given OLE in contrast to the TBI treatment group (p <0.05). This study found an elevated level of MMP9 expression in microglial cells in the brain tissue of a rat model of traumatic brain injury. In the context of traumatic brain injury, this pathway may be highly relevant as it is known to increase MMP-9 regulation [37-39]. According to our research, HMGB1 generated by dying cells after a stroke can swiftly attach to the nearby brain’s constitutively expressed TLR4 receptor, boosting MMP-9 regulation and causing more neurovascular damage and ischemic brain injury. RAGE, TLR2, and TLR4 are the three HMGB1 receptors that have been identified thus far. RAGE expression is low in the brain and is induced several hours after injury [40, 41]. Within three hours of the artery being blocked, the MMP-9 levels in the TLR4 mutant brain experience an ischemic reduction. A reduced brain damage volume cannot account for the decline in MMP-9 levels at this early stage because the brain infarct has not fully formed. According to some theories, TLR4 malfunction results in smaller infarcts and lower level of MMP-9 expression after a stroke and plays a role in the cell death that occurs as a result [42, 43]. Our research supports earlier findings and adds additional proof that extracellular HMGB1 activates TLR4 in a way that does not require TNF for MMP-9 to be upregulated. It is unclear whether the HMGB1-TLR4 pathway is involved in the regulation of other MMPs because mMMP-3 and other MMPs are also involved in ischemia-induced cell death [44]. The Effect of Oleuropein Administration and Administration Duration on Neuronal Cell Apoptosis in Brain Tissue with Traumatic Brain Injury Treatment Oleuropein, which is generally known as one of the most prominent phenolic compounds in olive plants, has been proven to have several pharmacological functions consisting of anticancer, antioxidant,anti- inflammatory, and antiviral properties. Previous studies have shown that oleuropein induces apoptosis activity and suppresses tumor growth in different cancer cells including hepatocellular carcinoma and promyelocytic leukemia [45, 47]. In this study, observation of apoptosis was conducted on neuronal cells, to explore the role of OLE in apoptosis occurrence. The technique used was the same as in the previous experiment, using immunohistochemistry to observe AIF and TUNEL assay for apoptosis. The results revealed a significantly lower level of AIF in the TBI group with OLE treatment compared to the TBI treatment group (p < 0.05). The results of the tunnel assay data calculation showed values that were in line with AIF expression, where OLE administration in the TBI model rats significantly reduced (p < 0.05) the number of neuronal cells undergoing apoptosis compared to rats that only received TBI treatment. In another study, it was also stated that by controlling the Akt/GSK-3b signalling system. In a classic rat model of IRI, Olive Leaf Extract (OLE) demonstrated enhanced neurological and cognitive outcomes, reduced brain edema, elevated levels of neurotrophic factors, and a lower incidence of neuronal cell apoptosis. OLE exhibited an anti-apoptotic response through anti-inflammatory actions, as well as suppression of lipid peroxidation and neutrophil infiltration in a rat model of spinal cord trauma. In addition, oral administration of OLE improved cerebral injury in a rat model of IRI by inhibiting Bax expression related to apoptosis, thus suppressing Bcl-2 activation [45]. Protein X promotes apoptosis in glioma cells by increasing caspase-3 and 9 expressions. Bcl2 and Bax regulate the mitochondrial apoptosis pathway, with Bcl2 being anti-apoptotic and Bax being pro-apoptotic. Ole increases the Bax/Bcl2 ratio, supporting apoptosis in various cancer cell lines, including MIA PaCa-2 pancreatic cancer cells. Ole affects the proapoptotic protein P53 and the Bax/bcl2 ratio, causing apoptosis in breast and colon tumors, but not in glioma cells. Ole also induces apoptosis in MIA PaCa-2 cancerous pancreatic cells through c-Jun and c-Fos dimerization [45, 46]. Ole (200 µM) promotes apoptosis in vitro in various cancer cells through different mechanisms such as dimerizing c-Jun and c-Fos into AP1 in MIA PaCa-2 cancerous pancreatic cells, increasing the pro-apoptotic potential in TCAM-2 and SEM-1 cells with BAX overexpression, and p38/ATF-2 pathway activation in NSCLC H1299 malignancies of the lung’s cells. Ole also decreased the expression of HIF-1α protein in HT-29 human colon adenocarcinoma cells. However, Ole at doses of 200 or 400 did not affect the p38, ERK, or JNK pathways in glioma cancer cells [47]. Furthermore, Oleuropein (0.04% of the diet) has been shown to inhibit
HIF-1α and adipogenesis in B16F10 melanoma cells in high-fat diet-fed rats, leading to the prevention of cancer development. BCL2-associated cell death agonist, which is activated by the Akt pathway, promotes apoptosis. At 500 µM for prostate cancer and 60 µM for HepG2 hepatocellular carcinoma cells, ole inhibits the Akt pathway in vitro . In vitro studies on HepG2 cells have demonstrated Ole’s (60 M) ability to inhibit the phosphatidylinositol 3-kinase (PI3K)/Akt/NF-kB and phosphatidylinositol 3-kinase (PI4K)/Akt/mammalian target rapamycin (mTOR) pathways. Ole (200/400 M) also inhibits the Akt pathway in vitro in glioma cells while also regulating the apoptosis-supporting enzymes Bax, Bcl2, MMP-2, and MMP-9 [48, 49]. Jnk’s dual pro- and anti-apoptotic behavior distorts its role in cancer depending on the type of the cells and what is activated or inhibited. Research shows that in the absence of active NF-kB, the Jnk pathway favours apoptosis. Although prolonged Jnk activation is still thought to cause apoptosis, the importance of Jnk activation in the survival and proliferation of many cancer cells is well known [50, 51].
Conclusion
In summary, here is an effect in oleuropein isolate administration to lower expression of Apoptosis Inducing Factor and Matrix Metalloproteinase-9 in rat model of traumatic brain injury. Therefore,Oleuropein has a potential neuroprotective effect in traumatic brain injury by reducing inflammation and apoptosis. Oleuropein can be considered as a potential therapeutic agent for traumatic brain injury in the future.
Acknowledgements
Ethical Approval
This study has been approved by our institution
Author contribution
Abdurrahman Mousa: Conceptualization, performing experiment, funding acquisition, writing original draft and preparation, and finishing. Ridha Dharmajaya: Conceptualization, supervision-writing, review, and editing. Julia Reveny: Supervision-writing and review. Khairul Putra Surbakti: Supervision-writing and editing. Hanif Gordang Tobing: Conceptualization, and review and editing.
Syafruddin Ilyas: Supervision. Rosita Juwita Sembiring: Cut. Aria Arina, Wibi Riawan: Performing experiments.
Disclosure Statement
The authors have nothing to disclose.References
| 1 | Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, Rosenfeld JV. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;130(4):1080-1097.
https://doi.org/10.3171/2017.10.JNS17352 |
| 2 | Li Q, Wang P, Huang C, Chen B, Liu J, Zhao M, Zhao J. N-Acetyl serotonin protects neural progenitor cells against oxidative stress-induced apoptosis and improves neurogenesis in adult mouse hippocampus following traumatic brain injury. J Mol Neurosci. 2019;67(4):574-588.
https://doi.org/10.1007/s12031-019-01263-6 |
| 3 | Glynn N, Agha A. The frequency and the diagnosis of pituitary dysfunction after traumatic brain injury. Pituitary. 2019;22(3):249-260.
https://doi.org/10.1007/s11102-019-00938-y |
| 4 | Stocchetti N, Carbonara M, Citerio G, Ercole A, Skrifvars MB, Smielewski P, Zoerle T, Menon DK. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16(6):452-464.
https://doi.org/10.1016/S1474-4422(17)30118-7 |
| 5 | Hale AC, Bohnert KM, Grekin R, Sripada RK. Traumatic brain injury in the general population: Incidence, mental health comorbidity, and functional impact. J Nerv Ment Dis. 2019;207(1):38-42.
https://doi.org/10.1097/NMD.0000000000000915 |
| 6 | Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
https://doi.org/10.1093/bja/aem131 |
| 7 | Cheng G, Kong RH, Zhang LM, Zhang JN. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br J Pharmacol. 2012;167(4):699-719.
https://doi.org/10.1111/j.1476-5381.2012.02025.x |
| 8 | Hu Q, Chen C, Khatibi NH, Li L, Yang L, Wang K, Han J, Duan W, Zhang JH, Zhou C. Lentivirus-mediated transfer of MMP-9 shRNA provides neuroprotection following focal ischemic brain injury in rats. Brain Res. 2011;1367:347-359.
https://doi.org/10.1016/j.brainres.2010.10.002 |
| 9 | Shao AW, Wu HJ, Chen S, Ammar AB, Zhang JM, Hong Y. Resveratrol attenuates early brain injury after subarachnoid hemorrhage through inhibition of NF-κB-dependent inflammatory/MMP-9 pathway. CNS Neurosci Ther. 2014;20(2):182.
https://doi.org/10.1111/cns.12194 |
| 10 | Jia F, Yin YH, Gao GY, Wang Y, Cen L, Jiang JY. MMP-9 inhibitor SB-3CT attenuates behavioral impairments and hippocampal loss after traumatic brain injury in rat. J Neurotrauma. 2014;31(13):1225-1234.
https://doi.org/10.1089/neu.2013.3230 |
| 11 | Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009;65(4):702-708.
https://doi.org/10.1227/01.NEU.0000351768.11363.48 |
| 12 | Omar SH. Oleuropein in olive and its pharmacological effects. Sci Pharm. 2010;78(2):133-154.
https://doi.org/10.3797/scipharm.0912-18 |
| 13 | Zhang W, Liu X, Li Q. Protective effects of oleuropein against cerebral ischemia/reperfusion by inhibiting neuronal apoptosis. Med Sci Monit. 2018;24:6587-6598. Available from: https://doi.org/10.12659/MSM.912336.
https://doi.org/10.12659/MSM.912336 |
| 14 | Hashmi MA, Khan A, Hanif M, Farooq U, Perveen S. Traditional uses, phytochemistry, and pharmacology of Olea europaea (olive). Evid Based Complement Alternat Med. 2015.
https://doi.org/10.1155/2015/541591 |
| 15 | Khalatbary AR, Ahmadvand H. Neuroprotective effect of oleuropein following spinal cord injury in rats. Neurol Res. 2012;34(1):44-51
https://doi.org/10.1179/1743132811Y.0000000058 |
| 16 | Mete M, Unsal U. Neuroprotective Effects of Oleocanthal, A Compound in Virgin Olive Oil, in A Rat Model of Traumatic Brain Injury. Turk Neurosurg. 2017;2(April 2018):1-8. doi: 10.5137/1019-5149.JTN.21417-17.2.
https://doi.org/10.5137/1019-5149.JTN.21417-17.2 |
| 17 | Notoatmodjo S. Metodologi Penelitian Kesehatan. Binarupa Aksara; 2003.
|
| 18 | Ba XH, Cai LP, Han W. Effect of cilostazol pretreatment on the PARP/APOPTOSIS INDUCING FACTOR-mediated apoptotic pathway in rat cerebral ischemia-reperfusion models. Exp Ther Med. 2014;7(5):1209-1214.
https://doi.org/10.3892/etm.2014.1551 |
| 19 | Lopez-Rodriguez AB, Acaz-Fonseca E, Viveros MP, Garcia-Segura LM. Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PLoS One. 2015;10(6).
https://doi.org/10.1371/journal.pone.0128782 |
| 20 | Chenna A, Petropoulos C, Winslow J. Highly Sensitive Single Molecular Array Immunoassay Measurement of t-Tau, NF-L, GFAP, and UCH-L1 Biomarkers in Acute Concussion/Mild Traumatic Brain Injury (mTBI) Patient Serum and Saliva Samples (P2. 9-047).
|
| 21 | Cruz-Haces M, Tang J, Acosta G, Fernandez J, Shi R. Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl Neurodegener. 2017. Available from: https://doi.org/10.1186/s40035-017-0088-2.
https://doi.org/10.1186/s40035-017-0088-2 |
| 22 | Sifringer M, Stefovska V, Zentner I, Hansen B, Stepulak A, Knaute C, Marzahn J, Ikonomidou C. The role of matrix metalloproteinases in infant traumatic brain injury. Neurobiol Dis. 2007;25(3):526-535. Available from: https://doi.org/10.1016/j.nbd.2006.10.019.
https://doi.org/10.1016/j.nbd.2006.10.019 |
| 23 | Simon DW, McGeachy MJ, Bayly RD, Clark RSB, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171-191. Available from: https://doi.org/10.1038/nrneurol.2017.13.
https://doi.org/10.1038/nrneurol.2017.13 |
| 24 | Ostrowski RP, Pucko E, Matyja E. The effectiveness of hyperbaric oxygen modalities against vascular component of traumatic brain injury. Brain Hemorrhages. 2020;118-123. Available from: https://doi.org/10.1016/j.hest.2020.04.002.
https://doi.org/10.1016/j.hest.2020.04.002 |
| 25 | Sowers JL, Sowers ML, Shavkunov AS, Hawkins BE, Wu P, DeWitt DS, Prough DS, Zhang K. Traumatic brain injury induces region-specific glutamate metabolism changes as measured by multiple mass spectrometry methods. iScience. 2021;24(10). Available from: https://doi.org/10.1016/j.isci.2021.103108.
https://doi.org/10.1016/j.isci.2021.103108 |
| 26 | Vaghebin R, Khalili M, Amiresmaili S, Roghani M, Esmaeili Saber SS, Namdar H. Saphenous vein phlebotomy alleviates neuroinflammatory response and oxidative stress following traumatic brain injury. Interdiscip Neurosurg. 2022;30. Available from: https://doi.org/10.1016/j.inat.2022.101626.
https://doi.org/10.1016/j.inat.2022.101626 |
| 27 | Nakashima M, Gotoh M, Hashimoto K, Endo M, Murakami-Murofushi K, Ikeshima-Kataoka H, Miyamoto Y. The neuroprotective function of 2-carba-cyclic phosphatidic acid: Implications for tenascin-C via astrocytes in traumatic brain injury. J Neuroimmunol. 2021;361. Available from: https://doi.org/10.1016/j.jneuroim.2021.577749.
https://doi.org/10.1016/j.jneuroim.2021.577749 |
| 28 | Borumand MR, Babaloii F, Mirmotahari SA, Maghsoudi AS, Torabi R, Mojtahedzadeh M, Norouzi P, Rad-Malekshahi M, Javar HA, Hassani S. Recent trends and innovations in biosensors development for biomarkers towards monitoring traumatic brain injury. Biosens Bioelectron X. 2022;12. Available from: https://doi.org/10.1016/j.biosx..
https://doi.org/10.1016/j.biosx.2022.100247 |
| 29 | Sezer C, Zırh S, Gokten M, Sezer A, Acıkalın R, Bilgin E, Zırh EB. Neuroprotective Effects of Milrinone on Acute Traumatic Brain Injury. World Neurosurgery [Preprint]. Available from: https://doi.org/10.1016/j.wneu.2022.11.072.
https://doi.org/10.1016/j.wneu.2022.11.072 |
| 30 | Rosyidi RM, Priyanto B, Islam AA, Hatta M, Bukhari A, Prihastomo KT, et al. Role of MLC901 in increasing neurogenesis in rats with traumatic brain injury. Ann Med Surg (Lond). 2020;60:36-40. Available from: https://doi.org/10.1016/j.amsu.2020.10.013.
https://doi.org/10.1016/j.amsu.2020.10.013 |
| 31 | Dai Y, Jin W, Cheng L, Yu C, Chen C, Ni H. Nur77 is a promoting factor in traumatic brain injury-induced nerve cell apoptosis. Biomed Pharmacother. 2018;108:774-782. Available from: https://doi.org/10.1016/j.biopha.2018.09.091.
https://doi.org/10.1016/j.biopha.2018.09.091 |
| 32 | Xu XJ, Yang MS, Zhang B, Niu F, Dong JQ, Liu BY. Glucose metabolism: A link between traumatic brain injury and Alzheimer's disease. Chin J Traumatol. 2021;24(1):5-10. Available from: https://doi.org/10.1016/j.cjtee.2020.10.001.
https://doi.org/10.1016/j.cjtee.2020.10.001 |
| 33 | Agrawal RR, Larrea D, Shi L, Song D, Xu Y, Yun TD, et al. Upregulated functionality of mitochondria-associated ER membranes in a mouse model of traumatic brain injury [Preprint]. Available from: https://doi.org/10.1101/2020.11.13.381756.
https://doi.org/10.1101/2020.11.13.381756 |
| 34 | Fan H, Duan H, Hao P, Gao Y, Zhao W, Hao F, et al. Cellular regeneration treatments for traumatic brain injury. Med Novel Technol Devices. 2022;16:100182. Available from: https://doi.org/10.1016/j.medntd.2022.100182.
https://doi.org/10.1016/j.medntd.2022.100182 |
| 35 | He M, Liu WG, Wen L, Du HG, Yin LC, Chen L. Influence of acute ethanol intoxication on neuronal apoptosis and Bcl-2 protein expression after severe traumatic brain injury in rats. Chin J Traumatol. 2013;16:136-139.
|
| 36 | Li X, Yu J, Ma D, Weng X. Edaravone improves the post-traumatic brain injury dysfunction in learning and memory by modulating Nrf2/ARE signal pathway. Clinics. 2021;76. Available from: https://doi.org/10.6061/clinics/2021/e3131.
https://doi.org/10.6061/clinics/2021/e3131 |
| 37 | Johnson NH, de Rivero Vaccari JP, Bramlett HM, Keane RW, Dietrich WD. Inflammasome activation in traumatic brain injury and Alzheimer's disease [Preprint]. Available from: https://doi.org/10.1016/j.trsl.2022.08.014.
https://doi.org/10.1016/j.trsl.2022.08.014 |
| 38 | Van den Bedem H, Kuhl E. Molecular mechanisms of chronic traumatic encephalopathy. Curr Opin Biomed Eng. 2017;23:23-30. Available from: https://doi.org/10.1016/j.cobme.2017.02.003.
https://doi.org/10.1016/j.cobme.2017.02.003 |
| 39 | Veenith T, Goon SS, Burnstein RM. Molecular mechanisms of traumatic brain injury: the missing link in management. World J Emerg Surg. 2009;4(1):7. Available from: https://doi.org/10.1186/1749-7922-4-7.
https://doi.org/10.1186/1749-7922-4-7 |
| 40 | Mendes Arent A, Souza LF, Walz R, Dafre AL. Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. BioMed Res Int. 2014. Available from: https://doi.org/10.1155/2014/723060.
https://doi.org/10.1155/2014/723060 |
| 41 | Zhai K, Duan H, Wang W, Zhao S, Khan GJ, Wang M, et al. Ginsenoside Rg1 ameliorates blood-brain barrier disruption and traumatic brain injury via attenuating macrophages derived exosomes miR-21 release. Acta Pharm Sin B. 2021;11(11):3493-3507. Available from: https://doi.org/10.1016/j.apsb.2021.03.032.
https://doi.org/10.1016/j.apsb.2021.03.032 |
| 42 | Xu M, Wang W, Lu W, Ling X, Rui Q, Ni H. Evodiamine prevents traumatic brain injury through inhibiting oxidative stress via PGK1/NRF2 pathway. Biomed Pharmacother. 2022;153:113435. Available from: https://doi.org/10.1016/j.biopha.2022.113435.
https://doi.org/10.1016/j.biopha.2022.113435 |
| 43 | Prins M, Greco T, Alexander D, Giza CC. The pathophysiology of traumatic brain injury at a glance. Dis Model Mech. 2013;6(6):1307-1315. Available from: https://doi.org/10.1242/dmm.011585.
https://doi.org/10.1242/dmm.011585 |
| 44 | Bossio S, Perri A, Malivindi R, Giordano F, Rago V, Mirabelli M, et al. Oleuropein Counteracts Both the Proliferation and Migration of Intra-and Extragonadal Seminoma Cells. Nutrients. 2022;14(11):2323. Available from: https://doi.org/10.3390/nu14112323.
https://doi.org/10.3390/nu14112323 |
| 45 | Rishmawi S, Haddad F, Dokmak G, Karaman R. A Comprehensive Review on the Anti-Cancer Effects of Oleuropein. Life. 2022;12(8):1140. Available from: https://doi.org/10.3390/life12081140.
https://doi.org/10.3390/life12081140 |
| 46 | Ma X, Niu X, Zhao J, Deng Z, Li J, Wu X, et al. Downregulation of Sepina3n Aggravated Blood-Brain Barrier Disruption after Traumatic Brain Injury by Activating Neutrophil Elastase in Mice. Neuroscience. 2022;503:45-57. Available from: https://doi.org/10.1016/j.neuroscience.2022.08.023.
https://doi.org/10.1016/j.neuroscience.2022.08.023 |
| 47 | Ho MH, Yen CH, Hsieh TH, Kao TJ, Chiu JY, Chiang YH, et al. CCL5 via GPX1 activation protects hippocampal memory function after mild traumatic brain injury. Redox Biol. 2021;46:102067. Available from: https://doi.org/10.1016/j.redox.2021.102067.
https://doi.org/10.1016/j.redox.2021.102067 |
| 48 | Tajik M, Danielli E, Sharma B, Noseworthy MD. A review of molecular and genetic factors for determining mild traumatic brain injury severity and recovery. Brain Disord. 2022:100058. Available from: https://doi.org/10.1016/j.dscb.2022.100058.
https://doi.org/10.1016/j.dscb.2022.100058 |
| 49 | Zhang S, Wu X, Wang J, Shi Y, Hu Q, Cui W, et al. Adiponectin/AdiopR1 signaling prevents mitochondrial dysfunction and oxidative injury after traumatic brain injury in a SIRT3 dependent manner. Redox Biol. 2022;54:102390. Available from: https://doi.org/10.1016/j.redox.2022.102390.
https://doi.org/10.1016/j.redox.2022.102390 |
| 50 | Zhang W, Liu X, Li Q. Protective effects of oleuropein against cerebral ischemia/reperfusion by inhibiting neuronal apoptosis. Med Sci Monit. 2018;24:6587-6598. Available from: https://doi.org/10.12659/MSM.912336.
https://doi.org/10.12659/MSM.912336 |
| 51 | Omagari K, Kato S, Tsuneyama K, Hatta H, Sato M, Hamasaki M, et al. Olive leaf extract prevents spontaneous occurrence of non-alcoholic steatohepatitis in SHR/NDmcr-cp rats. Pathology. 2010;42:66-72.
https://doi.org/10.3109/00313020903434389 |