Hybrid Organic-Inorganic Copper and Cobalt Complexes for Antimicrobial Potential Applications
bBasic & Applied Scientific Research Center, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, 31441, Dammam, Saudi Arabia,
cDepartment of Biology, College of Science, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, 31441, Dammam, Saudi Arabia,
dFaculty of Science, Biochemistry Department, King Abdulaziz University, Saudi Arabia
Keywords
Abstract
Background/Aims:
The naturally occurring phenolic chemical curcumin (CUR), which was derived from the Curcuma longa plant, has a variety of biological actions, including anti-inflammatory, antimicrobial, antioxidant, and anticancer activities. Curcumin is known for its restricted bioavailability due to its hydrophobicity, poor intestinal absorption, and quick metabolism. To boost the biological effects of these bioactive molecules, it is necessary to raise both their bioavailability and their solubility in water. Aim: The aim of this study is to synthesize and characterize hybrid organic-inorganic complexes of copper and cobalt, and to evaluate their antimicrobial potential against a range of pathogenic microorganisms.Methods:
The synthesis of metal curcumin complexes (Cu-CUR and Co-CUR) was achieved by mixing curcumin with copper acetate monohydrate. The solid residue was isolated, filtered, and dried in an oven. X-ray diffraction analysis was used to identify the structure and phase of the prepared samples. FTIR spectra were recorded using a Shimadzu 2200 module. The antimicrobial activity of the prepared complexes was evaluated against four bacterial strains and two Candida species. The chemical materials were dissolved in DMSO to a final concentration of 20%, and the plates were incubated at 37°C for 24 hours. The results showed that the prepared complexes had antimicrobial activity against the tested microorganisms.Results:
The study compared the Powder X-ray diffraction (XRD) patterns of prepared copper and cobalt complexes to pure curcumin, revealing new, isostructural complexes. The FTIR analysis showed that the Cu-CUR and Co-CUR complexes varied in their inhibitory effect against microorganisms, with Co-CUR being more effective. The results are consistent with previous studies showing the cobalt-curcumin complex was effective against various bacterial genera, with inhibition activity varying depending on the species and strains of microorganisms.Conclusion:
Copper and cobalt curcumin complexes, synthesized at room temperature, exhibit high crystallinity and antimicrobial activity. Co-CUR, with its superior antibacterial potential, outperforms pure curcumin in inhibiting microbes. Further investigation is needed to understand their interaction mechanisms with bacteria and fungi.Introduction
Curcumin, which is known as diferuloylmethane and identified by the IUPAC as (1E,6E)-1, 7-bis(4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5-dione. It, is a yellow chemical found in plants of the Curcuma longa as shown in Fig. 1 [1].
Fig. 1: The Chemical Structure of Curcumin (top) and The Turmeric plant (Curcuminoids).
Curcumin is the main curcuminoid in turmeric (Curcuma longa ), which belongs to the ginger family, Zingiberaceae. This ingredient is sold as an herbal supplement, cosmetic ingredient, flavoring agent, and food colorant (Akbar et al., 2018) [2].
Turmeric is said to have been widely utilized for medicinal treatments of many illnesses. The main active component of turmeric, orange-yellow curcumin, is principally responsible for its significance in medicinal therapy. Turmeric powder contains 2-5% curcumin, a lipophilic polyphenol substance [2, 3].
Curcumin research has revealed that the chemical structure of this polyphenol compound has antibacterial, antioxidant, anti-inflammatory, antiplatelet aggregation, antiangiogenic, and antimutagenic characteristics [3-5].
Curcumin has been shown to provide preventative and preventive effects against several illnesses, including autoimmune, cancer, neurological, liver, lung, metabolic, and cardiovascular diseases (CVDs), due to its characteristics. Polyphenol compounds have received a great deal of attention recently because of their effects on a variety of degenerative disorders, including cancer [4, 6, 7].
Curcumin has a low bioavailability in the body and its use is restricted due to poor absorption by the body, rapid excretion, and rapid metabolism. Curcumin has not been marketed as a medicinal medicine, mostly because of the pharmacokinetic restrictions of curcumin, which makes its anti-inflammatory impact in clinical use less than optimal. When curcumin is taken orally, the majority of it is eliminated with metabolites and only a tiny quantity enters the bloodstream for use. This amount is far less than what is needed to inhibit most of the curcumin’s anti-inflammatory targets [8, 9].
Curcumin might be categorized as a class 4 substance by the Biopharmaceutics Classification System (BCS) due to its low water solubility and restricted permeability across intestinal membranes. The poor water solubility and sensitivity of curcumin to alkaline conditions, heat, light, enzymes, oxygen, and ascorbic acid, among other things, frequently restricts its practical usage. In this way, curcumin is a prospective option for the creation of novel natural materials, to improve its stability against the above-mentioned causes [10, 11].
Some techniques are being researched to improve curcumin’s bioavailability. These techniques include using curcumin nanoparticles, phospholipid complexes, liposomal curcumin, and complexation of curcumin with metal ions. Several complexes of transitional metal ions such as VO (IV), Cu (II), Co (III), Fe (III), Pt (II), and Nd (III) with curcumin and a variety of co-ligands have been prepared and all these complexes are potential photochemotherapeutic [1, 12-14].
Some metals have a reputation for having antibacterial properties, and in some situations, they can be used as powerful therapeutic agents to treat bacterial illnesses. One of the significant trace elements that is less toxic than non-essential metals and is considered a necessary element for people is cobalt. Occasional studies on the antibacterial effects of cobalt complexes have been published, with Co (II) complexes receiving the most attention, perhaps because of their aqueous stability, availability, and simplicity of production. It has also been demonstrated that the Co (III)-curcumin complex is biologically more potent against cancer than free curcumin [15-19].
It has been demonstrated that cobalt-curcumin has stronger antibacterial properties than either cobalt alone or curcumin alone. Another study of the Co (II) curcumin complex showed a modest antibacterial action toward B. subtilis, P. aeruginosa, and S. aureus. Where the β-diketone moiety of curcumin generally interacts with copper (II) to form curcumin- copper (II) complexes [16, 20, 21].
Curcumin creates complexes of type 1:1 and 1:2 when combined with copper. Curcumin-copper (II) complexes have been demonstrated that they have more antioxidant action than curcumin. As a result, the overall structure and the physical-chemical characteristics of curcumin are changed as a result of the interaction of copper with its ligand, which improves the biological potency of curcumin. where Copper (Cu) is an essential element that is redox-active, present in many tissues, and is important for the efficient operation of several biological processes [5, 22, 23].
It has been demonstrated that the Curcumin-copper (II) complexes have higher superoxide scavenging activities than free curcumin. Additionally, Curcumin-copper (II) complexes enhance the activities of antioxidant enzymes, such as catalase, superoxide dismutase, and glutathione peroxidase so the Curcumin-copper (II) complexes have a positive effect. Moreover, the Curcumin-copper (II) complexes combination demonstrated the strongest antibacterial activity, as well as the most effective anthelmintic activity against Pheretima posthuma [24, 25].
Also, Tthe Curcumin-copper (II) complexes combination demonstrated greater antifungal activity than curcumin alone, Aspergillus flavus and Penicillium digitatum were used as test subjects for antifungal activity. Also, the antimicrobial activity of the Curcumin-copper (II) complexes combination was investigated against S. aureus, E. Coli, P. aeruginosa, B. subtilis, A. flavus , and C. albicans . However, it does not exhibit much microbial inhibition. The curcumin-copper (II) complexes combination has also been suggested to be developed into a vaginal microbicidal gel against viral infections due to its good microbicidal activity. Also, the antiviral activity of a Curcumin-copper (II) complex was demonstrated against multiple viruses, including herpes simplex virus strains, vesicular stomatitis virus, and vaccine virus. As a result, the Cu-curcumin complex has desirable antiviral activity against a variety of viruses [26-28].
Due to the potent association between copper and curcumin, prior research has shown the significance of curcumin-copper (II) complexes in DNA damage because of the strong interaction between curcumin and copper (II). The pro-oxidant properties of Curcumin-copper (II) complexes, on the other hand, are demonstrated by the production of reactive oxygen species at high levels of free copper in a reducing atmosphere, this situation causes cancer cells to experience DNA damage and the inhibition of vital signaling pathways, which causes apoptosis. In essence, curcumin plays a dual role in the presence of copper, acting as both an antioxidant and a prooxidant. These interesting phenomena considerably contribute to the wide range of therapeutic actions of curcumin [29, 30].
Materials and Methods
Synthesis of CUR Complexes
Synthesis of metal curcumin complexes (Cu-CUR‑ and Co-CUR) was prepared by mixing curcumin (0.5 g) and copper acetate monohydrate Cu (CH₃COO)2.H2O (0.338 g) or Co(CH3CO2)2·4H2O (0.271 g) in ethanol (20 mL) and stirred, at ambient temperature, using ultrasonic stirring machine for 6 h. The obtained solid residue was isolated by filtration and dried in an oven at 60°C for 24h.
Analytical instruments
The structure and phase identification of the prepared samples were carried out using X–ray diffraction analysis device (PanAlytical MPDPRO diffractometer) equipped with CuKα radiation (λKα1/α2 = 1.540560/1.544330Å). X–ray diffraction patterns are obtained in the 2θ range of 10–60 °, the step size is 0.02 and the time per step is 30 s.
FTIR spectra were recorded by using spectrochemical studies based on Fourier-transform infrared (FTIR) (Shimadzu 2200), in the 4000-400 cm-1 region, module (IR SPIRIL-T), Serial Number (A22415801447AE).
Biological methods
Screening for antimicrobial activity. Two different metal curcumin complexes prepared Co-CUR and Cu-CUR were evaluated for their antimicrobial activity at a concentration of 0.6 g/ml (W/V) the chemical materials were dissolved in 3 ml dimethyl sulfoxide (DMSO) to a final concentration of 20%, using agar well diffusion technique (Wayne, 1999) against four bacterial strains and two Candida species they included; Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC25922, and Staphylococcus epidermidis ATCC 12228, Staphylococcus aureus ATCC 25923, Candida tropicalis ATCC 13803 and Candida albicans ATCC 14053 all microorganisms were provided from King Fahd Hospital in Al Khobar city – Saudi Arabia. Inoculums of the microbes were prepared from an overnight culture grown in Nutrient Broth and microbial turbidity was adjusted between 0.63-1.8×108 CFU/ml which represented 0.5- 0.63 McFarland standards, using Biomerieux DensiCHEK plus meter device. Half melilite from pre-adjusted microbial inoculum was transferred induvial to Petri plates and about fifteen ml of permeated Mueller-Hinton Agar media was poured gently over the microbial inoculums, the plates were rotated carefully to ensure an even distribution of the microbial inoculums, then cultures were left at room temperature to solidify for 2-5 minutes. Three wells size 8mm were made in each Petri plate using a sterile cork-borer, and then 100 μL of each chemical sample was transferred directly to the wells individually. DMSO was used as a negative control, and antibiotic erythromycin (CN10µg) and nystatin (100 mg) were used as positive controls for the bacteria and fungi, respectively. Plates were kept in the refrigerator for one hour to allow better diffusion for the chemicals in the culture agar media. Then plates were incubated at 37 °C for 24 h, and the clear area without microbial growth around the well was measured using a digital caliper.
Results
XRD analysis
The Powder X-ray diffraction (XRD) patterns of the prepared copper and cobalt complexes are given in Fig. 2. These patterns are compared to the X-ray pattern of pure curcumin on one hand and checked with the international crystal database (ICDD) on the other hand. This comparison leads to the conclusion that the prepared complexes are new since no known phase has been identified. Furthermore, copper and cobalt complexes seem to be isostructural since their X-ray patterns are almost identical.
The crystallite size ‘D’ is determined from XRD diffracted patterns through the Scherrer equation
$${ D = \frac{K \lambda}{\beta \cos \theta}}$$
[37, 38] where, \(K = 0.89\) is a constant, \( \lambda = 1.5406 \unicode{xC5} \), \(\theta \) and \( \beta \) are the diffraction angle and the corresponding full width at half-maximum (FWHM) of the observed peak, respectively. the calculated sizes are given in Table 1. These values show that pure curcumin and cobalt and copper complexes are at the nanometric scale. The contribution of the amorphous and crystalline phases was investigated by the XRD method [37, 39, 40] to determine the Crystallinity Index (CI).
$${ CI \% = \frac{\text{crystalline peak area}}{\text{crystalline and amorphous area}} \times 100 }$$
The CI calculated values indicate an increase from the pure curcumin to the prepared copper and cobalt curcumin complexes.
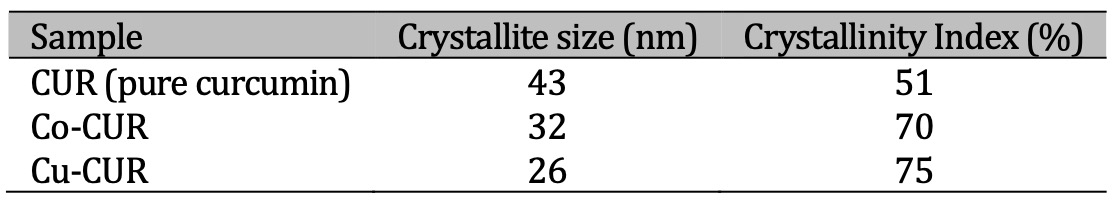
Table 1: Crystallite size and crystallinity index for the prepared samples
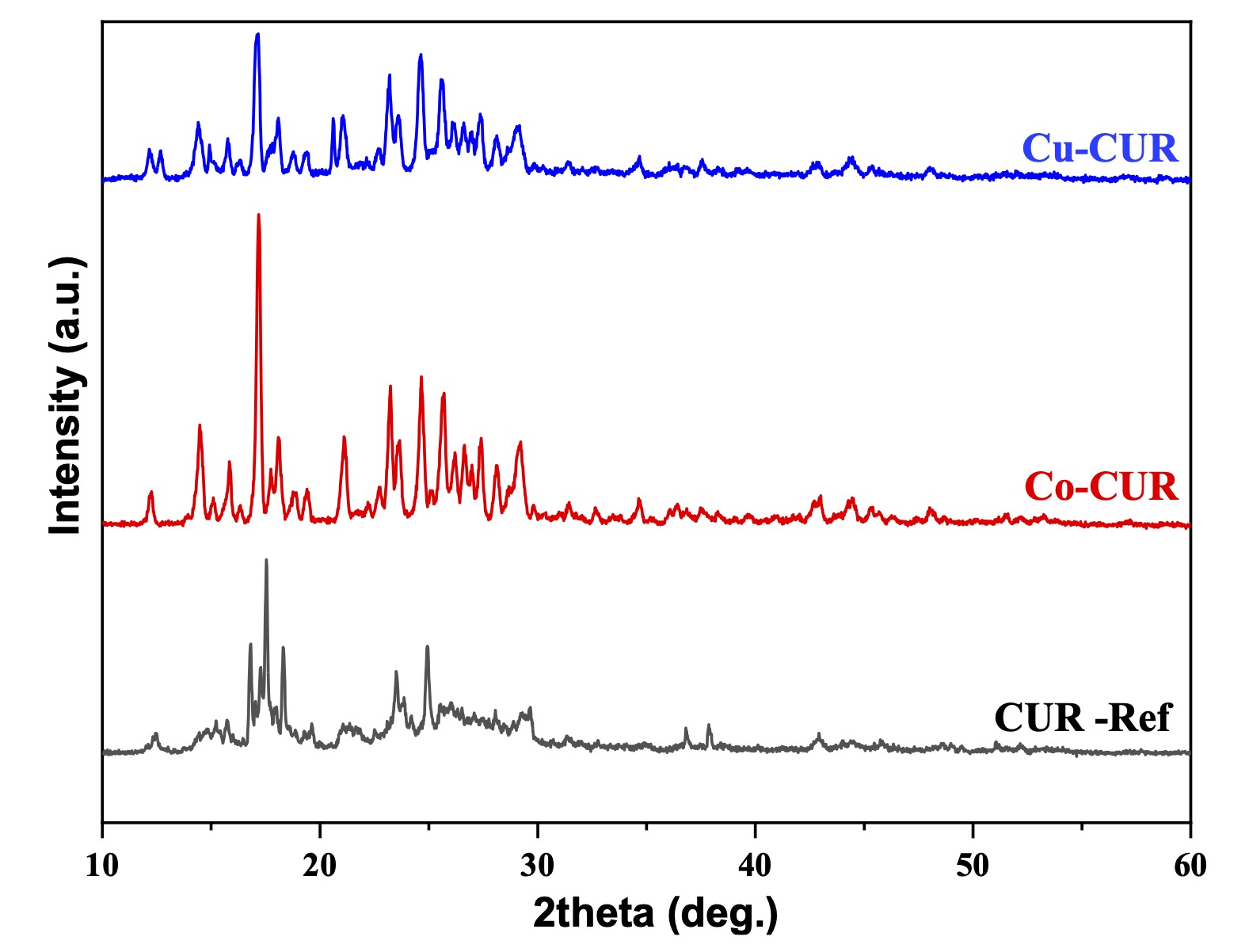
Fig. 2: X-ray diffraction patterns of pure Curcumin (as a reference), cobalt, and copper curcumin complexes (2θ Degree).
FTIR analysis
The FTIR spectrum of pure Curcumin (as a reference), cobalt, and copper curcumin complexes are given in Fig. 3.
O-H Vibrations: Band absorption due to stretching vibrations of hydroxyl groups are observed around 3520 cm-1. The in-plane deformation vibration and the out-of-plane vibration of hydroxyl groups appear in the regions 1440-1260 cm-1 and 700-600 cm-1, respectively. The bands observed around 3550 and 1430 cm-1 have been assigned to OH stretching and OH in-plane deformation vibrations, respectively [31].
C=O Stretching vibrations: The absorption bands corresponding to stretching vibrations of C=O bonds give paramount structural information. It occurs as a very strong band and is easily recognized. C=O stretching vibration was observed in the region 1850-1450 cm-1. In this region, the bands observed at 1515, 1453, and 1421 cm-1 have been assigned to carbonyl stretching vibrations. All the observed bands corresponding to C-C, C=C, and C-H appeared at approximately the same frequencies except those of the C=O group which were shifted to lower frequency indicating the formation of bridged bonds C=O-Cu-O=C in the case of Cu-CUR and Co-CUR samples.
C-O Stretching vibrations: The presence of bands corresponding to alcohol C-O groups is proof that Cu or Co were bonded to the curcumin via oxygen of carbonyl groups. The stretching vibration corresponding to alcohol C-O groups is observed normally in the region 1200-1000 cm-1. A strong band and a medium-strong band observed at 1275 and 1230 cm-1, respectively in all spectra have been assigned to C-O stretching vibrations. The assignments are in good agreement with the literature [31].
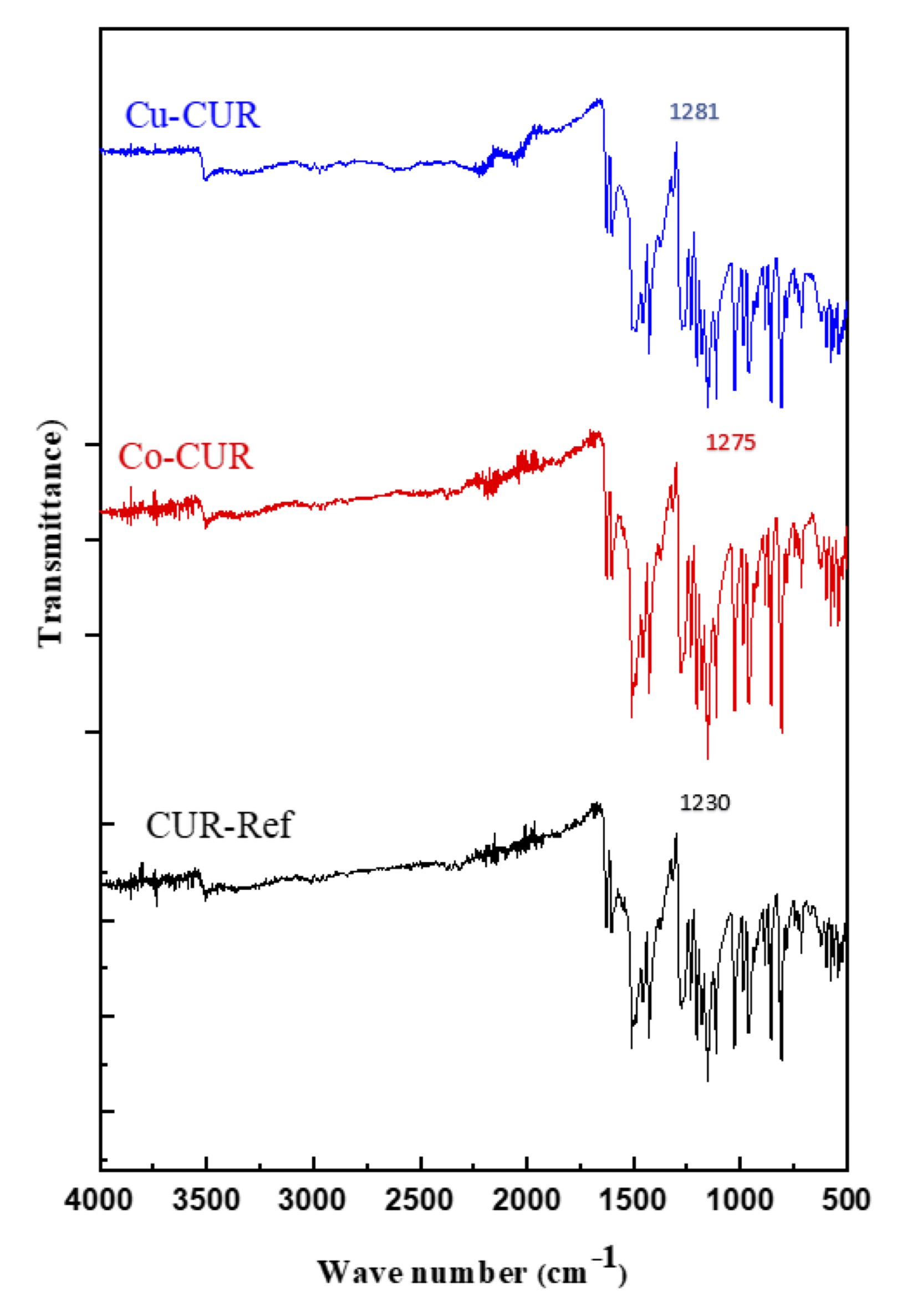
Fig. 3: FTIR spectra of pure Curcumin (as a reference), cobalt, and copper curcumin complexes.
Biological activity study
Curcumin, an active ingredient, has attracted a lot of interest as a plant-based substance with pharmacological characteristics that are pleiotropic, antioxidant, Anti-inflammatory, hypoglycaemic, neuroprotective, immunomodulatory and antimicrobial. However, curcumin’s poor water solubility, low serum levels, restricted tissue distribution, quick metabolism, and excretion limit its effectiveness as a medicinal and limit its bioavailability, for these purposes, the complexes of cobalt or copper with curcumin were considered (Fig. 4) [5, 26, 32].
Our results showed that the tested Cu-CUR and Co-CUR varied in their inhibitory effect against microorganisms. In general, the Cu-CUR and Co-CUR can inhibit microorganisms, this may be due to the high crystallinity index, which ranged from 70% to 75% for Co-CUR and Cu-CUR, respectively, compared to the crystallinity index for pure curcumin, which was 51% (Table 1).
Furthermore, the Co-CUR was more effective than Cu-CUR as the Co-CUR was able to inhibit all six test microbes at different levels. Whereas Cu-CUR was only effective against both Gram-positive bacteria (Staph. aureus ATCC 25923 and Staph . epidermidis ATCC 12228) and P. aeruginosa ATCC 27853 bacterium while the E. coli and fungi were resisters. Moreover, gram-positive bacteria showed more sensitivity to both chemicals than gram-negative bacteria (Table 2, Fig. 5 and 6). This may be attributed to the fact that cobalt has better magnetic properties than the copper compound, which would help the cobalt adhere better than the copper compound to the surfaces of microorganisms, as the surfaces of bacteria have negative charges. These negative charges increase on the surfaces of Gram-positive bacteria because of the presence of teichoic acids in the peptidoglycan layer compared to Gram-negative bacteria [33].
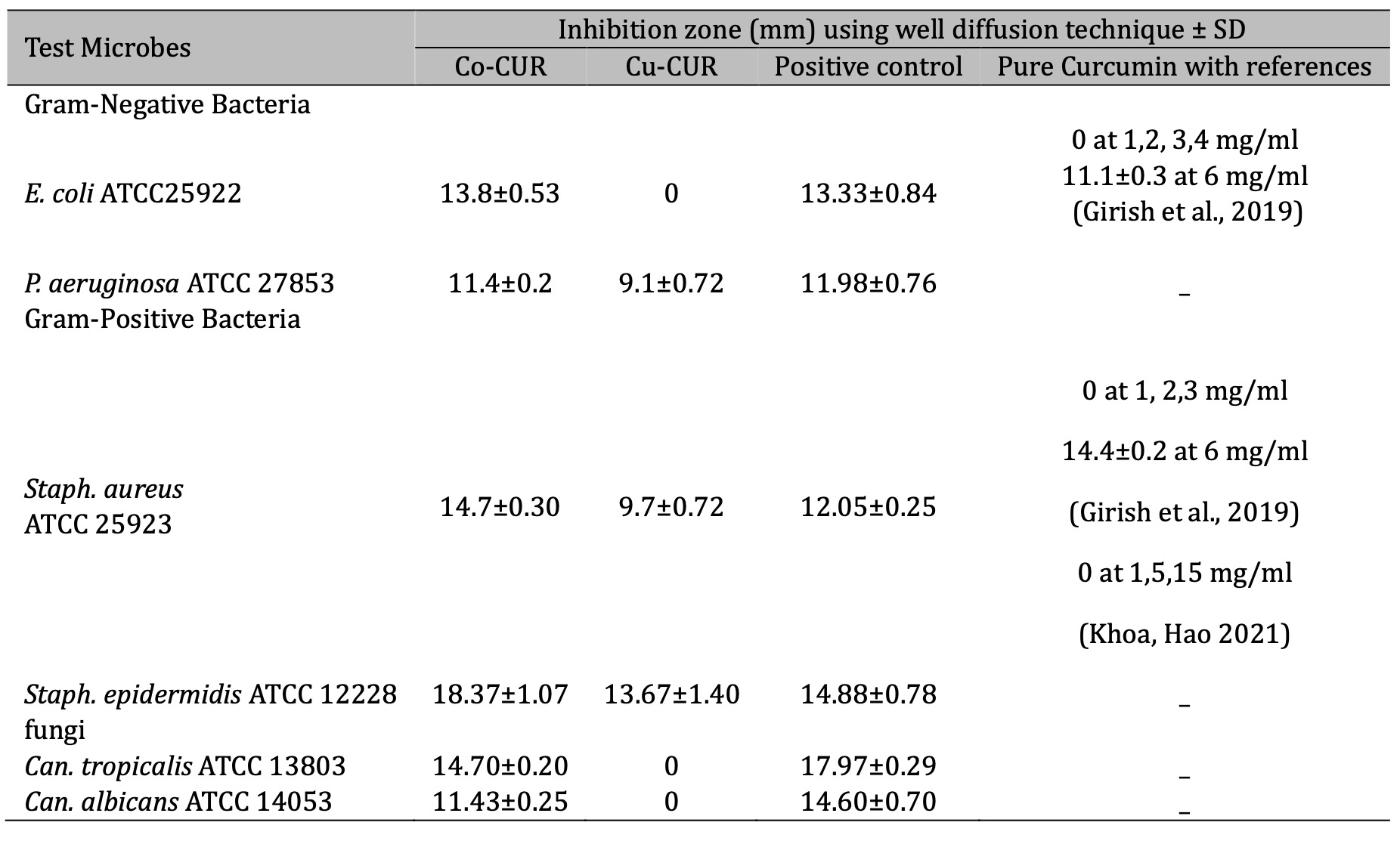
Table 2: Antimicrobial activity of Co-CUR and Cu-CUR at 600 mg/ml, against pathogenic microorganisms using agar well diffusion technique, compared with pure curcumin in reference studies. * Negative control was DMSO showed zero activity
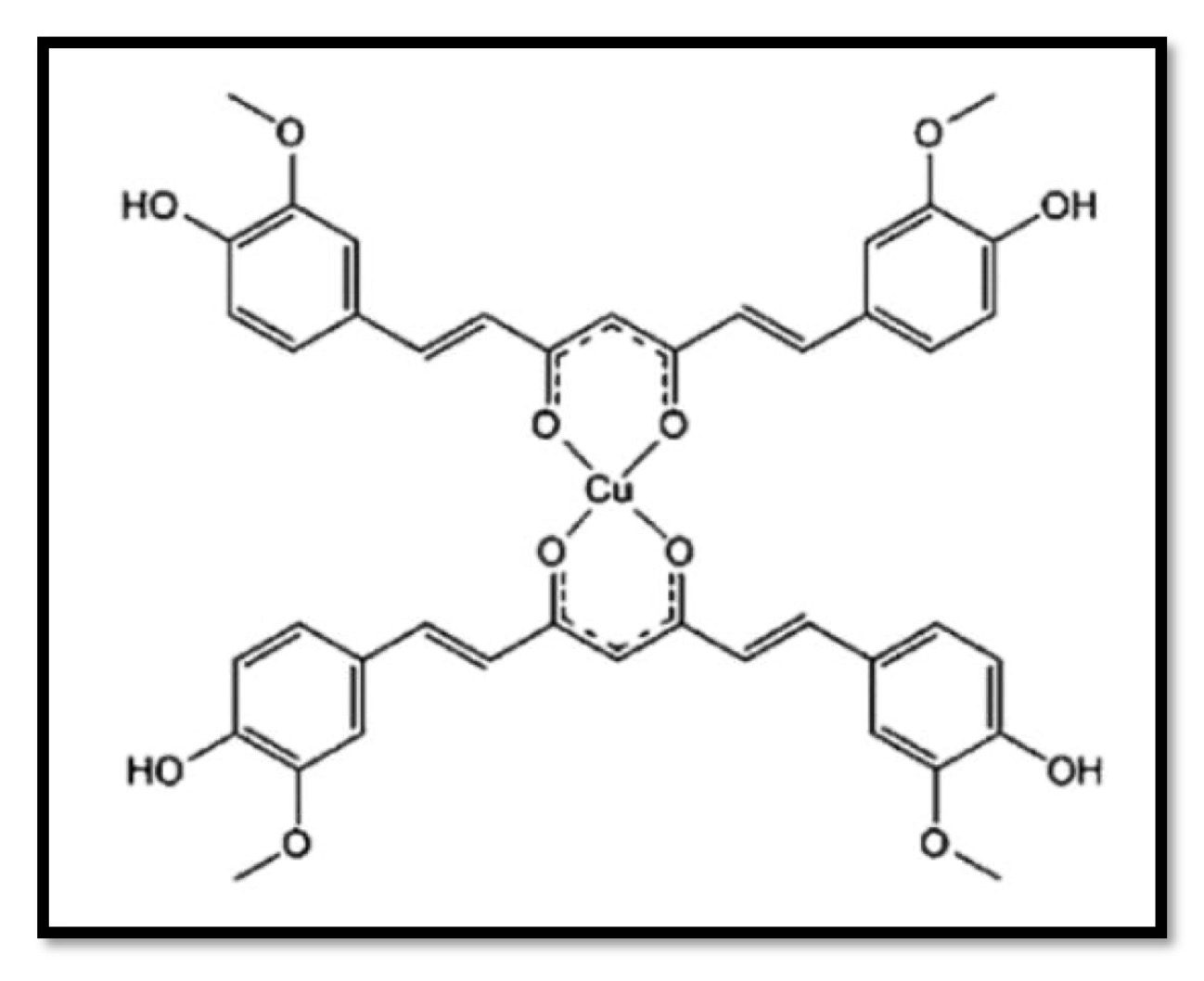
Fig. 4: The expected structure of curcumin copper (or cobalt) complexes.
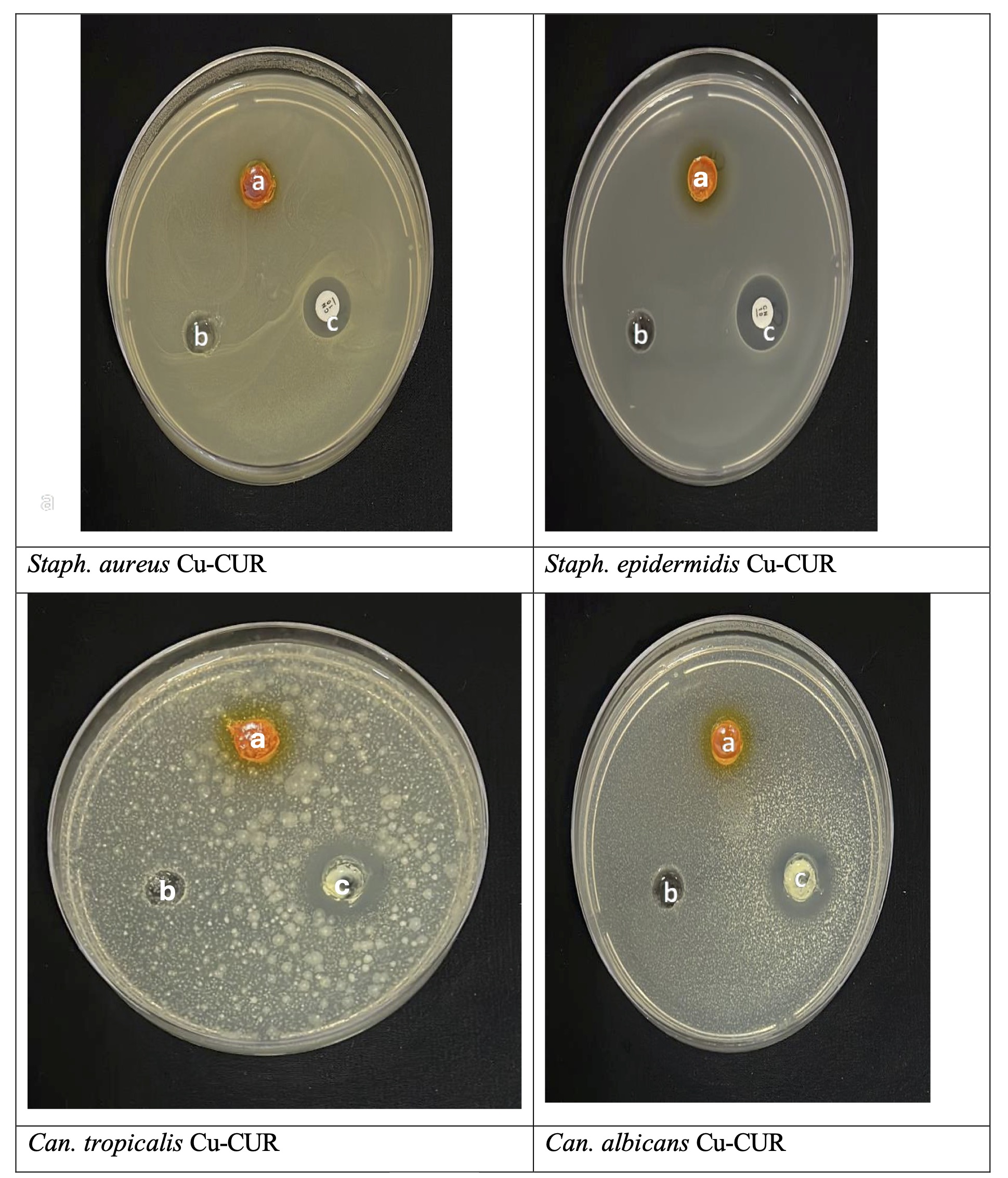
Fig. 5: Antimicrobial activity of Cu-CUR, against pathogenic microorganisms using agar well diffusion technique: (a) Cu-CUR, (b) negative control DMSO, (c) Positive control.
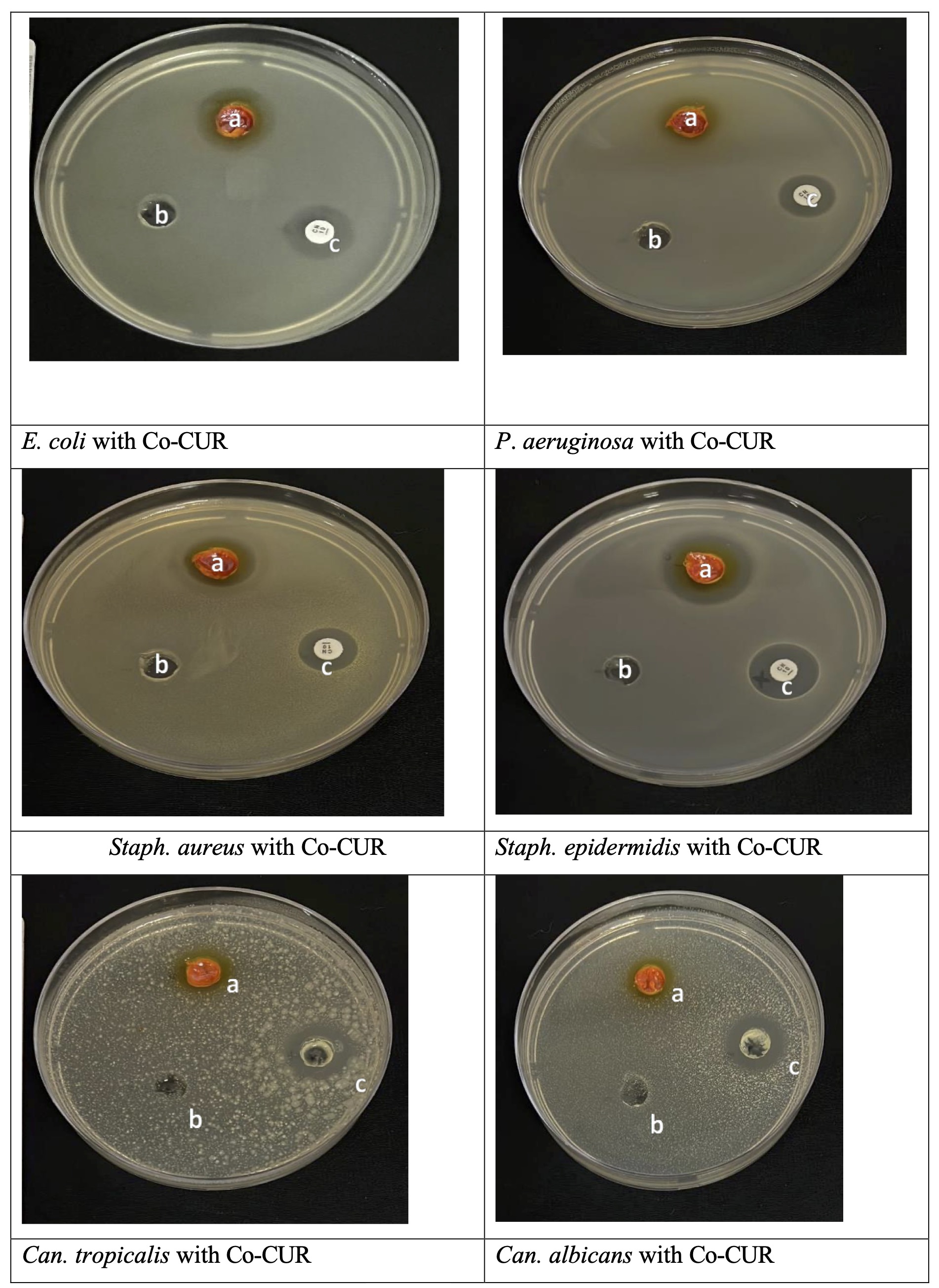
Fig. 6: Antimicrobial activity of Co-CUR, against pathogenic microorganisms using agar well diffusion technique: (a) Co-CUR (b) negative control DMSO, (c) Positive control.
Discussion
The high susceptibility of Gram-positive bacteria to the tested materials may be because they do not contain an outer membrane that may play a role in protecting the bacterial cell from chemicals, the inhibitory activity of tested chemicals may relate to their ability to penetrate the cell wall of the microbes, additionally, another study showed Gram-positive bacteria exhibit a substantially higher level of sensitivity than Gram-negative bacteria, where the hydrophilic lipopolysaccharides that are abundant in gram-negative bacteria’s outer membrane serve as a barrier to the efficient penetration of a range of hydrophobic antibacterial substances . Moreover, the results showed that the inhibitory effect of the Co-CUR. E.coli and Staph aureus which may make this substance a good alternative to some antibiotics with weak or non-existent effects [26].
Our current results are consistent with a study conducted by Girish et al. 2019 where they showed that the cobalt-curcumin complex was effective against numerous bacterial genera like Escherichia coli, Bacillus subtilis, Klebsiella pneumoniae, Shigella flexneri, Salmonella typhi, Staphylococcus aureus, and Proteus vulgaris, and the inhibition activity related to the concentrations as they found that low concentrations do not show antibacterial activity and the Co(II) curcumin were higher than Curcumin. Moreover, our results agree with a recent study by researchers Hoa and Hoang 2021 that copper (II)-curcumin complex can inhibit the growth of Staph. aureus ATCC 6538 while the pure curcumin had no inhibitory effect at concentrations of 1, 5,15 mg/mL so we can conclude that pure Curcumin has a weak or non-ability to inhibit bacterial [34, 35].
Adamczak et al., 2020, found that curcumin had weak antimicrobial activity against several pathogenic clinical microbes. It was also found that curcumin showed poor activity against clinical Candida spp. and it showed good inhibition activity against some genera, and they concluded that the effectiveness of curcumin varies significantly depending on the species and strains of microorganism. Another study by Chandrasekar et al., 2014, found that curcumin requires higher concentrations to inhibit the bacteria Staph. aureus , Bacillus subtilis , E. coli , P. aeruginosa , and Salmonella typhi , comparing to curcumin Co and Cu complexes. Therefore, scientists are currently seeking to modify the structure of curcumin with metals, this may improve its solubility in water such a process may increase uptake by microbes leading to increases in its inhibitory ability [26].
Conclusion
Copper and cobalt curcumin complexes have been synthesized at room temperature and were characterized by X-ray diffraction and Infrared spectroscopy. The prepared complexes have a nanometric scale since no heat treatment was applied in the synthesis method. The obtained complexes are characterized by high crystallinity compared to curcumin. The prepared metal curcumin complexes Co-CUR and Cu-CUR were evaluated for their antimicrobial activity at a concentration of 0.6 g/ml (W/V). The obtained results showed that the two curcumin complexes were capable of inhibiting the microbes’ growth, possibly due to their high percentage of crystallinity index (70% - Co-CUR), (75% Cu-CUR) compared to the pure curcumin (51%). The Co-CUR had a higher ability to inhibit all six isolates than the Cu-CUR. This may be related to its magnetic properties, which increase its ability to adhere to the surfaces of bacteria and Candida. Our results indicated that Co-CUR exhibited superior antibacterial potential compared to the antibiotic against E. coli and Staph. aureus , Hence, Co-CUR can be considered a good alternative to some antibiotics with weak or non-existent effects.
The present study shows promising outcomes concerning copper and cobalt curcumin complexes. Further investigation of the biological importance of these metal complexes is needed to understand the mechanism of interaction with bacteria and fungi. Moreover, in-vitro and in-vivo studies are needed for the successful development and applications of prepared complexes.
Acknowledgements
We would like to thank the College of Science at Imam Abdulrahman bin Faisal University, especially the Department of Chemistry and Department of Biology, for their support and for their efforts to extend a helping hand to us at all times.
Disclosure Statement
There are no conflicts of interest.
References
| 1 | Liu Z, Smart JD, Pannala AS: Recent developments in formulation design for improving oral bioavailability of curcumin: a review. Journal of drug delivery science and technology 2020;60:102082.
https://doi.org/10.1016/j.jddst.2020.102082 |
| 2 | Akbar MU, Rehman K, Zia KM, Qadir MI, Akash MSH, Ibrahim M: Critical review on curcumin as a therapeutic agent: From traditional herbal medicine to an ideal therapeutic agent. Critical Reviews™ in Eukaryotic Gene Expression 2018;28
https://doi.org/10.1615/CritRevEukaryotGeneExpr.2018020088 |
| 3 | Deogade SC, Ghate S: Curcumin: therapeutic applications in systemic and oral health. Int J Biol Pharm Res 2015;6:281-290.
|
| 4 | Prasad S, Gupta SC, Tyagi AK, Aggarwal BB: Curcumin, a component of golden spice: from bedside to bench and back. Biotechnology advances 2014;32:1053-1064.
https://doi.org/10.1016/j.biotechadv.2014.04.004 |
| 5 | Shehzad A, Rehman G, Lee YS: Curcumin in inflammatory diseases. Biofactors 2013;39:69-77.
https://doi.org/10.1002/biof.1066 |
| 6 | Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB: Multitargeting by turmeric, the golden spice: From kitchen to clinic. Molecular nutrition & food research 2013;57:1510-1528.
https://doi.org/10.1002/mnfr.201100741 |
| 7 | Sohrab G, Hosseinpour-Niazi S, Hejazi J, Yuzbashian E, Mirmiran P, Azizi F: Dietary polyphenols and metabolic syndrome among Iranian adults. International journal of food sciences and nutrition 2013;64:661-667.
https://doi.org/10.3109/09637486.2013.787397 |
| 8 | Kocaadam B, Şanlier N: Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Critical reviews in food science and nutrition 2017;57:2889-2895.
https://doi.org/10.1080/10408398.2015.1077195 |
| 9 | Peng Y, Ao M, Dong B, Jiang Y, Yu L, Chen Z, Hu C, Xu R: Anti-inflammatory effects of curcumin in the inflammatory diseases: status, limitations and countermeasures. Drug design, development and therapy 2021:4503-4525.
https://doi.org/10.2147/DDDT.S327378 |
| 10 | Paolino D, Vero A, Cosco D, Pecora TM, Cianciolo S, Fresta M, Pignatello R: Improvement of oral bioavailability of curcumin upon microencapsulation with methacrylic copolymers. Frontiers in Pharmacology 2016;7:485.
https://doi.org/10.3389/fphar.2016.00485 |
| 11 | da Silva AC, de Freitas Santos PD, do Prado Silva JT, Leimann FV, Bracht L, Goncalves OH: Impact of curcumin nanoformulation on its antimicrobial activity. Trends in Food Science & Technology 2018;72:74-82.
https://doi.org/10.1016/j.tifs.2017.12.004 |
| 12 | Mitra K, Gautam S, Kondaiah P, Chakravarty AR: Platinum (II) complexes of curcumin showing photocytotoxicity in visible light. European Journal of Inorganic Chemistry 2017;2017:1753-1763.
https://doi.org/10.1002/ejic.201601078 |
| 13 | Bhattacharyya U, Kumar B, Garai A, Bhattacharyya A, Kumar A, Banerjee S, Kondaiah P, Chakravarty AR: Curcumin "drug" stabilized in oxidovanadium (IV)-BODIPY conjugates for mitochondria-targeted photocytotoxicity. Inorganic chemistry 2017;56:12457-12468.
https://doi.org/10.1021/acs.inorgchem.7b01924 |
| 14 | Deka B, Bhattacharyya A, Mukherjee S, Sarkar T, Soni K, Banerjee S, Saikia KK, Deka S, Hussain A: Ferrocene conjugated copper (II) complexes of terpyridine and traditional Chinese medicine (TCM) anticancer ligands showing selective toxicity towards cancer cells. Applied Organometallic Chemistry 2018;32:e4287.
https://doi.org/10.1002/aoc.4287 |
| 15 | Yuan P, Ding X, Yang YY, Xu QH: Metal nanoparticles for diagnosis and therapy of bacterial infection. Advanced Healthcare Materials 2018;7:1701392.
https://doi.org/10.1002/adhm.201701392 |
| 16 | Hatamie S, Nouri M, Karandikar S, Kulkarni A, Dhole S, Phase D, Kale S: Complexes of cobalt nanoparticles and polyfunctional curcumin as antimicrobial agents. Materials Science and Engineering: C 2012;32:92-97.
https://doi.org/10.1016/j.msec.2011.10.002 |
| 17 | Chang EL, Simmers C, Knight DA: Cobalt complexes as antiviral and antibacterial agents. Pharmaceuticals 2010;3:1711-1728.
https://doi.org/10.3390/ph3061711 |
| 18 | Garai A, Pant I, Banerjee S, Banik B, Kondaiah P, Chakravarty AR: Photorelease and cellular delivery of mitocurcumin from its cytotoxic cobalt (III) complex in visible light. Inorganic Chemistry 2016;55:6027-6035.
https://doi.org/10.1021/acs.inorgchem.6b00554 |
| 19 | Banerjee S, Chakravarty AR: Metal complexes of curcumin for cellular imaging, targeting, and photoinduced anticancer activity. Accounts of chemical research 2015;48:2075-2083.
https://doi.org/10.1021/acs.accounts.5b00127 |
| 20 | Refat MS: Synthesis and characterization of ligational behavior of curcumin drug towards some transition metal ions: Chelation effect on their thermal stability and biological activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2013;105:326-337.
https://doi.org/10.1016/j.saa.2012.12.041 |
| 21 | Prasad S, DuBourdieu D, Srivastava A, Kumar P, Lall R: Metal-curcumin complexes in therapeutics: an approach to enhance pharmacological effects of curcumin. International journal of molecular sciences 2021;22:7094.
https://doi.org/10.3390/ijms22137094 |
| 22 | Călinescu M, Fiastru M, Bala D, Mihailciuc C, Negreanu-Pîrjol T, Jurcă B: Synthesis, characterization, electrochemical behavior and antioxidant activity of new copper (II) coordination compounds with curcumin derivatives. Journal of Saudi Chemical Society 2019;23:817-827.
https://doi.org/10.1016/j.jscs.2019.02.006 |
| 23 | Sarawi WS, Alhusaini AM, Fadda LM, Alomar HA, Albaker AB, Aljrboa AS, Alotaibi AM, Hasan IH, Mahmoud AM: Nano-curcumin prevents cardiac injury, oxidative stress and inflammation, and modulates TLR4/NF-κB and MAPK signaling in copper sulfate-intoxicated rats. Antioxidants 2021;10:1414.
https://doi.org/10.3390/antiox10091414 |
| 24 | Yan F-S, Sun J-L, Xie W-H, Shen L, Ji H-F: Neuroprotective effects and mechanisms of curcumin-Cu (II) and-Zn (II) complexes systems and their pharmacological implications. Nutrients 2017;10:28.
https://doi.org/10.3390/nu10010028 |
| 25 | Kareem A, Arshad M, Nami SA, Nishat N: Herbo-mineral based Schiff base ligand and its metal complexes: Synthesis, characterization, catalytic potential and biological applications. Journal of Photochemistry and Photobiology B: Biology 2016;160:163-171.
https://doi.org/10.1016/j.jphotobiol.2016.03.030 |
| 26 | Shakeri A, Panahi Y, Johnston TP, Sahebkar A: Biological properties of metal complexes of curcumin. BioFactors 2019;45:304-317.
https://doi.org/10.1002/biof.1504 |
| 27 | Gholami M, Zeighami H, Bikas R, Heidari A, Rafiee F, Haghi F: Inhibitory activity of metal-curcumin complexes on quorum sensing related virulence factors of Pseudomonas aeruginosa PAO1. AMB Express 2020;10:1-10.
https://doi.org/10.1186/s13568-020-01045-z |
| 28 | Chauhan G, Rath G, Goyal AK: In-vitro anti-viral screening and cytotoxicity evaluation of copper-curcumin complex. Artificial cells, nanomedicine, and biotechnology 2013;41:276-281.
https://doi.org/10.3109/21691401.2012.742096 |
| 29 | HM Leung M, Harada T, W Kee T: Delivery of curcumin and medicinal effects of the copper (II)-curcumin complexes. Current Pharmaceutical Design 2013;19:2070-2083.
https://doi.org/10.2174/138161213805289237 |
| 30 | Shrestha RM, Mahiya K, Shrestha A, Mohanty SR, Yadav SK, Yadav PN: Synthesis, characterization, anticancer, pharmacokinetics and molecular docking investigation of N (3)-alkyl incorporated-3-acetyl-4-hydroxycoumarin thiosemicarbazones and their copper (II) complexes. Journal of Molecular Structure 2024;1299:136945.
https://doi.org/10.1016/j.molstruc.2023.136945 |
| 31 | Mangolim CS, Moriwaki C, Nogueira AC, Sato F, Baesso ML, Neto AM, Matioli G: Curcumin-β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food chemistry 2014;153:361-370.
https://doi.org/10.1016/j.foodchem.2013.12.067 |
| 32 | Dai C, Lin J, Li H, Shen Z, Wang Y, Velkov T, Shen J: The natural product curcumin as an antibacterial agent: Current achievements and problems. Antioxidants 2022;11:459.
https://doi.org/10.3390/antiox11030459 |
| 33 | Sarwar A, Katas H, Samsudin SN, Zin NM: Regioselective sequential modification of chitosan via azide-alkyne click reaction: synthesis, characterization, and antimicrobial activity of chitosan derivatives and nanoparticles. PLoS One 2015;10:e0123084.
https://doi.org/10.1371/journal.pone.0123084 |
| 34 | Hao HM: Synthesis, characterization and evaluation of antibacterial activity of copper (II)-curcumin complex against staphylococcus aureus. Journal of Technical Education Science 2021:52-57.
https://doi.org/10.54644/jte.67.2021.1089 |
| 35 | Girish K, Channu B, BABA D: Synthesis and Antibacterial Activity of Cobalt (II) Complex of Curcumin. Indian Journal of Pharmaceutical Sciences 2019;81.
https://doi.org/10.4172/pharmaceutical-sciences.1000491 |