Lemon Juice and Peel Constituents Potently Stabilize Rat Peritoneal Mast Cells
aMiyagi University, School of Nursing, 1-1 Gakuen, Taiwa-cho, Kurokawa-gun, Miyagi, Japan
Keywords
Abstract
Background/Aims:
Lemons (Citrus limon) contain various nutrients and are among the most popular citrus fruit. Besides their antioxidant, anticancer, antibacterial, and anti-inflammatory properties, clinical studies have indicated their anti-allergic properties.Methods:
Using the differential-interference contrast (DIC) microscopy, we examined the effects of lemon juice and peel constituents, such as citric acid, ascorbic acid, hesperetin and eriodictyol, on the degranulation from rat peritoneal mast cells. Using fluorescence imaging with a water-soluble dye, Lucifer Yellow, we also examined their effects on the deformation of the plasma membrane.Results:
Lemon juice dose-dependently decreased the number of degranulated mast cells. At concentrations equal to or higher than 0.25 mM, citric acid, hesperetin, and eriodictyol significantly reduced the number of degranulating mast cells in a dose-dependent manner, while ascorbic acid required much higher doses to exert significant effects. At 1 mM, citric acid, hesperetin, and eriodictyol almost completely inhibited exocytosis and washed out the Lucifer Yellow trapped on the mast cell surface, while ascorbic acid did not.Conclusion:
This study provides in vitro evidence for the first time that lemon constituents, such as citric acid, hesperetin, and eriodictyol, potently exert mast cell-stabilizing properties. These properties are attributable to their inhibitory effects on plasma membrane deformation in degranulating mast cells.Introduction
Lemons (Citrus limon) are among the world’s most popular citrus fruit used in a variety of food preparations. Previous studies have revealed their health promoting functions, including anti-oxidant, anti-cancer, anti-bacterial, and anti-inflammatory properties [1-3]. In humans, lemon extracts have been reported to ameliorate the symptoms of allergic rhinitis [4-6], indicting the anti-allergic potential of lemons and identifying a new pharmacological avenue. Lemons are full of various nutrients, including flavonoids, vitamins, minerals, soluble or insoluble dietary fiber, essential oils, organic acids, and carotenoids [7]. Among them, lemon juice most abundantly contains citric acid followed by ascorbic acid (vitamin C), while lemon peel contains various flavonoids, mainly hesperidin and eriocitrin [8]. The anti-allergic properties of citric acid, hesperetin, and eriodictyol (active metabolites of hesperidin and eriocitrin, respectively) have been demonstrated in animal models of allergic diseases, such as atopic dermatitis and anaphylaxis [9-11]. However, little is known about the precise mechanisms underlying their anti-allergic properties.
In allergic reactions, mast cells release secretory granules, including chemical mediators, such as histamine, serotonin, leukotrienes and prostaglandins, in an exocytotic manner [12]. Most anti-allergic drugs exert their effects by antagonizing histamine H1 receptors in peripheral tissues [13]. However, several drugs or natural compounds exert stronger anti-allergic properties by directly inhibiting the process of exocytosis and thus stabilizing mast cells [14]. In our previous studies, by continuously monitoring the process of exocytosis in mast cells, we have provided in vitro evidence that adrenaline, macrolide antibiotics, corticosteroids, anti-hypertensives, and anti-allergic drugs exert mast cell-stabilizing properties [15-20]. Recently, we have additionally shown that food constituents, such as caffeine, catechin, and vitamins also stabilize mast cells [21, 22]. In the present study, to determine the anti-allergic properties of lemon juice and peel constituents and elucidate the underlying physiological mechanisms, we directly examined their effects on rat peritoneal mast cell degranulation. Here, we provide in vitro evidence for the first time that the lemon constituents and their active metabolites, such as citric acid, hesperetin, and eriodictyol, potently exert mast cell-stabilizing properties. Such properties may be attributable to their inhibitory effects on plasma membrane deformation in degranulating mast cells.
Materials and Methods
Cell Sources and Preparation
Male Wistar rats, outbred albino rats currently the most widely used for laboratory research [23], no less than 25 weeks old were purchased from The Jackson Laboratory Japan, Inc. (Yokohama, Japan). Wistar rats have originally and frequently been used for the isolation of mast cells [24, 25]. Mast cells isolated from rats of this age range were viable enough to be easily induced exocytosis by the exogenous pharmacological stimuli [15-19, 22, 26]. Owing to the differences in sex hormones [27], mast cells in females tend to be more hypersensitive than those in males. Therefore, we used only male rats throughout the experiments. Rats were anaesthetized with isoflurane and euthanized via cervical dislocation as permitted by euthanasia guidelines for adult laboratory rodents [28]. Animal protocols were approved by the Animal Care and Use Committee of Miyagi University (No. 2024-02). As previously described [15-22, 29], rat peritoneum was washed with a standard external (bathing) solution, comprising: NaCl, 145 mM; KCl, 4.0 mM; CaCl2, 1.0 mM; MgCl2, 2.0 mM; HEPES, 5.0 mM; bovine serum albumin, 0.01 % (pH 7.2 adjusted with NaOH); and isolated mast cells from the peritoneal cavity. The isolated mast cells were maintained in the external solution at room temperature (22-24℃) for approximately 8 h until use. The mast cell suspension, which was approximately 200/mL, was spread in a chamber placed at the head stage of an inverted microscope (Nikon, Tokyo, Japan). Mast cells were easily distinguished from other cell types by their characteristic intracellular secretory granules [15-22, 29]. The viability of mast cells was determined by their capacity to release secretory granules in response to external stimuli (Fig. 1Ab vs. a) and morphological intactness under differential-interference contrast (DIC) microscopy, as previously demonstrated [30, 31].
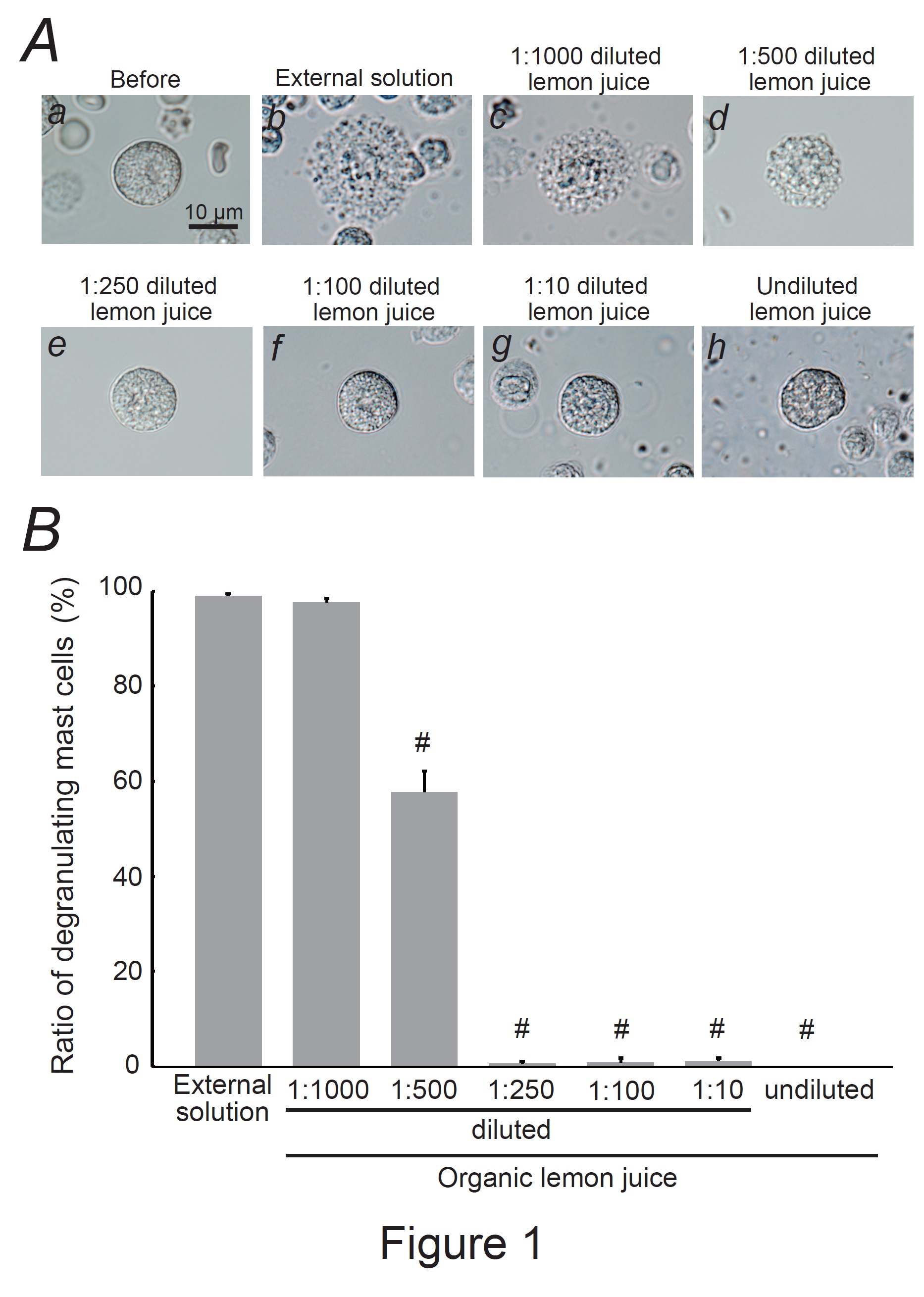
Fig. 1: Effects of organic lemon juice on mast cell degranulation. A: Differential-interference contrast (DIC) microscopic images were taken before (a) and after exocytosis was externally induced by compound 48/80 in mast cells incubated in the external solutions containing no substance (b) or 1:1000 diluted lemon juice (c), 1:500 diluted (d), 1:250 diluted (e), 1:100 diluted (f), 1:10 diluted (g), and undiluted lemon juice (h). B: After the mast cells were incubated in the external solutions containing no lemon juice or different dilutions of lemon juice, exocytosis was induced by compound 48/80. From a single rat, several samples of mast cell suspension were obtained from the peritoneal cavity. The aliquot of the sample was spread in a chamber placed at the head stage of an inverted microscope. Then bright-field images were obtained from randomly chosen 0.1-mm2 fields of view, in which 30-40 mast cells were evenly observed per field. The degranulating mast cells were expressed as the average percentages of the total mast cells in the 10 bright fields. # p<0.05 vs. incubation in the external solution alone. Values were presented as the means ± SEM. Differences were analyzed using ANOVA followed by Dunnett’s t-test. The experiments were repeated at least three times using three different rats to confirm the reproducibility of the data.
Quantification of Mast Cell Degranulation
Organic lemon juice (100% pure) was purchased from commercial sources (BIOCA, Nagano, Japan) and serially diluted with an external solution. Citric acid, hesperetin (Wako Pure Chemical Industries, Osaka, Japan), and eriodictyol (Extrasynthese, Lyon, France) were separately dissolved in an external solution to final concentrations of 0.1, 0.25, 0.5, and 1 mM. L(+)-ascorbic acid (Wako Pure Chem Ind.) was dissolved to final concentrations of 1, 2.5, 5, and 10 mM. In humans, the serum concentrations of citric acid, ascorbic acid, hesperetin and eriodictyol reach around 100-150 μM, 50-70 μM, 2-5 μM, 10-15 μM, respectively, after oral administration of physiological doses [32-34]. However, concentrations up to 0.5-1 mM were required for citric acid, hesperetin, and eriodictyol to elicit their pharmacological properties in cultured cell lines in vitro [35-37]. In contrast, ascorbic acid required approximately 10 times higher doses than citric acid, hesperetin, and eriodictyol (up to 5-10 mM) [22, 38, 39]. Therefore, the present study employed doses of 0.1-1 mM for citric acid, hesperetin, and eriodictyol, and 1-10 mM for ascorbic acid. After incubating mast cells with these solutions or the external solution alone, exocytosis was externally induced using compound 48/80 (Sigma-Aldrich Co., St. Louis, MO, USA; final concentration, 10 mg/mL) [15-22, 29]. We used rat-derived mast cells in our experiments as they are more responsive to compound 48/80 than those isolated from the mouse peritoneal cavity [40]. We obtained bright-field images from randomly chosen 0.1-mm2 fields of view (10 views from each condition), as previously described [15-22, 29]. We counted the number of degranulated mast cells (defined as cells surrounded by over eight granules outside the cell membrane) and calculated their ratio to the total number of mast cells.
Lucifer Yellow Trapping on Cell Subsurface
After mast cells were incubated with external solutions containing no substances, 1 mM citric acid, ascorbic acid, hesperetin, or eriodictyol for 10 min, exocytosis was externally induced using compound 48/80 (10 mg/mL). The cells were then incubated for 5 min at room temperature in an external solution containing the hydrophilic fluorescent, Lucifer Yellow dye [15, 16, 18, 20, 29, 41, 42] (Wako, Osaka, Japan; final concentration, 10 mM) and washed thoroughly three times with dye-free external solutions. Fluorescence images were captured using a TE 2000-E Nikon Eclipse fluorescence microscope (Nikon, Tokyo, Japan). Because Lucifer Yellow is a water-soluble fluorescent dye retained in the invaginated folds created in the plasma membranes [15, 18, 20, 41, 42], negative staining distinguishes substances that inhibit the membrane surface deformation of degranulating mast cells.
Statistical Analysis
Data were analyzed using Microsoft Excel (Microsoft Corporation, Redmond, WA., USA) and reported as means ± SEM. Statistical significance was assessed using ANOVA. Statistical significance was set at p < 0.05.
Results
Effects of organic lemon juice on mast cell degranulation
Mast cells incubated with the external solution demonstrated numerous wrinkles on the cell surface and the release of secretory granules due to exocytosis (Fig. 1Ab vs. a). However, exocytosis was partially or almost completely absent in mast cells incubated with 100 % (undiluted) lemon juice or serial dilutions (Fig. 1Ad-h). Quantitatively, a 1:1,000 dilution did not affect the number of degranulating mast cells (Fig. 1B); however, a 1:500 dilution significantly decreased the number of degranulating mast cells (external solution, 98.9 ± 0.59 % vs. 1:500 dilution, 57.7 ± 4.45 %; n=10, P<0.05), and the dilutions equal to or thicker than 1:250 almost totally suppressed degranulation (Fig. 1B). From these results, lemon juice was shown to inhibit exocytosis in a dose-dependent manner and thus exert mast cell-stabilizing properties.
Effects of citric acid and ascorbic acid on mast cell degranulation
As citric and ascorbic acids are major components of lemon juice [7, 43], we directly examined their effects on mast cell degranulation (Fig. 2). A relatively low citric acid concentration (0.1 mM) did not affect mast cell degranulation (Fig. 2Ac vs. b); the number of degranulated cells was comparable to that of cells incubated with the external solution alone (Fig. 2B). However, concentrations equal to or higher than 0.25 mM partially or entirely halted the process of exocytosis (Fig. 2Ad-f). Quantitatively, 0.25 mM citric acid significantly reduced the number of degranulating mast cells (external solution, 95.4 ± 2.08 % vs. 0.25 mM, 85.7 ± 1.58 %; n=10, P<0.05; Fig. 2B). Concentrations of 0.5 and 1 mM demonstrated a marked reduction, almost totally suppressing degranulation (0.5 mM, 1.93 ± 0.85 %; 1 mM, 0.45 ± 0.34 %; n=10, P<0.05; Fig. 2B). Consistent with our previous findings [22], relatively higher ascorbic acid concentrations (2.5, 5 and 10 mM) almost completely halted the process of exocytosis (Fig. 2Ah-j) and decreased the numbers of degranulating mast cells (external solution, 99.1 ± 0.43 % vs. 2.5 mM, 1.11 ± 0.74 %; 5 mM, 0.53 ± 0.53 %; 10 mM, 0.32 ± 0.24 %; n=10, P<0.05; Fig. 2C). However, approximately 10 times higher doses than citric acid were required to obtain similar suppressive effects on mast cell degranulation (Fig. 2C vs. B). These results indicated that lemon juice constituents, such as citric and ascorbic acids, dose-dependently inhibited the process of exocytosis and thus exerted mast cell-stabilizing properties.
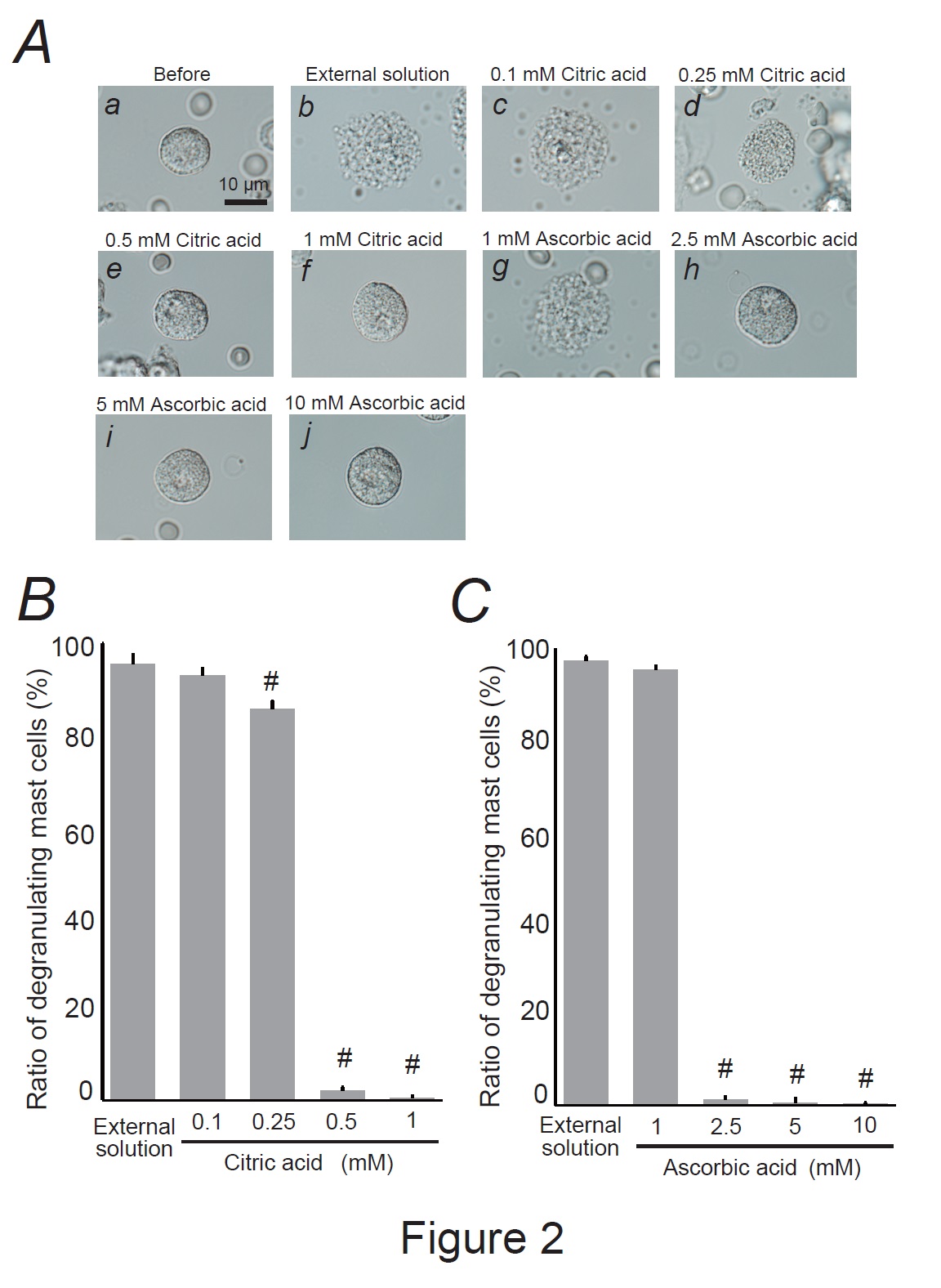
Fig. 2: Effects of citric acid and ascorbic acid on mast cell degranulation. A: Differential-interference contrast (DIC) microscopic images were taken before (a) and after exocytosis was externally induced by compound 48/80 in mast cells incubated in the external solutions containing no substances (b), 0.1 mM citric acid (c), 0.25 mM citric acid (d), 0.5 mM citric acid (e), 1 mM citric acid (f), 1 mM ascorbic acid (g), 2.5 mM ascorbic acid (h), 5 mM ascorbic acid (i), and 10 mM ascorbic acid (j). Effects of different concentrations of citric acid (0.1, 0.25, 0.5, and 1 mM) (B) and ascorbic acid (1, 2.5, 5, and 10 mM) (C). After the mast cells were incubated in the external solutions containing no substances or either substance, exocytosis was induced by compound 48/80. From a single rat, several samples of mast cell suspension were obtained from the peritoneal cavity. The aliquot of the sample was spread in a chamber placed at the head stage of an inverted microscope. Then bright-field images were obtained from randomly chosen 0.1-mm2 fields of view, in which 30-40 mast cells were evenly observed per field. The degranulating mast cells were expressed as the average percentages of the total mast cells in the 10 bright fields. # p<0.05 vs. incubation in the external solution alone. Values were presented as the means ± SEM. Differences were analyzed by ANOVA followed by Dunnett’s t-test. The experiments were repeated at least three times using three different rats to confirm the reproducibility of the data.
Effects of hesperetin and eriodictyol on mast cell degranulation
Hesperidin and eriocitrin are the main flavonoids in lemon peels [44]. Since they are metabolized into their aglycone forms by the gut microbiota, such as hesperetin and eriodictyol [45], we examined their direct effects on mast cell degranulation (Fig. 3). Similar to the effects of citric acid (Fig. 2A and B), a relatively low hesperetin concentration (0.1 mM) did not affect mast cell degranulation (Fig. 3Ac vs. b), and the number of degranulating cells was comparable to that of cells incubated with the external solution alone (Fig. 3B). However, concentrations equal to or higher than 0.25 mM partially or entirely halted the process of exocytosis (Fig. 3Ad-f). Quantitatively, 0.25 mM hesperetin significantly reduced the number of degranulating mast cells (external solution, 99.6 ± 0.43 % vs. 0.25 mM, 55.6 ± 4.89 %; n=10, P<0.05; Fig. 3B), with 0.5 and 1 mM showing even greater reductions, almost totally suppressing the numbers of degranulating mast cells (0.5 mM, 1.52 ± 0.67 %; 1 mM, 0.93 ± 0.65 %; n=10, P<0.05; Fig. 3B). Similar to the effects of hesperetin (Fig. 3Ad-f and B), eriodictyol at concentrations equal to or higher than 0.25 mM partially or almost entirely halted the process of exocytosis (Fig. 3Ah-j) and significantly suppressed the numbers of degranulating mast cells dose-dependently (external solution, 98.4 ± 1.06 % vs. 0.25 mM, 86.2 ± 2.34 %; 0.5 mM, 31.8 ± 2.82 %; 1 mM, 7.14 ± 1.51 %; n=10, P<0.05; Fig. 3C). These results indicated that hesperetin and eriodictyol, the active metabolites of lemon peel constituents, dose-dependently inhibited the process of exocytosis and thus exerted mast cell-stabilizing properties.
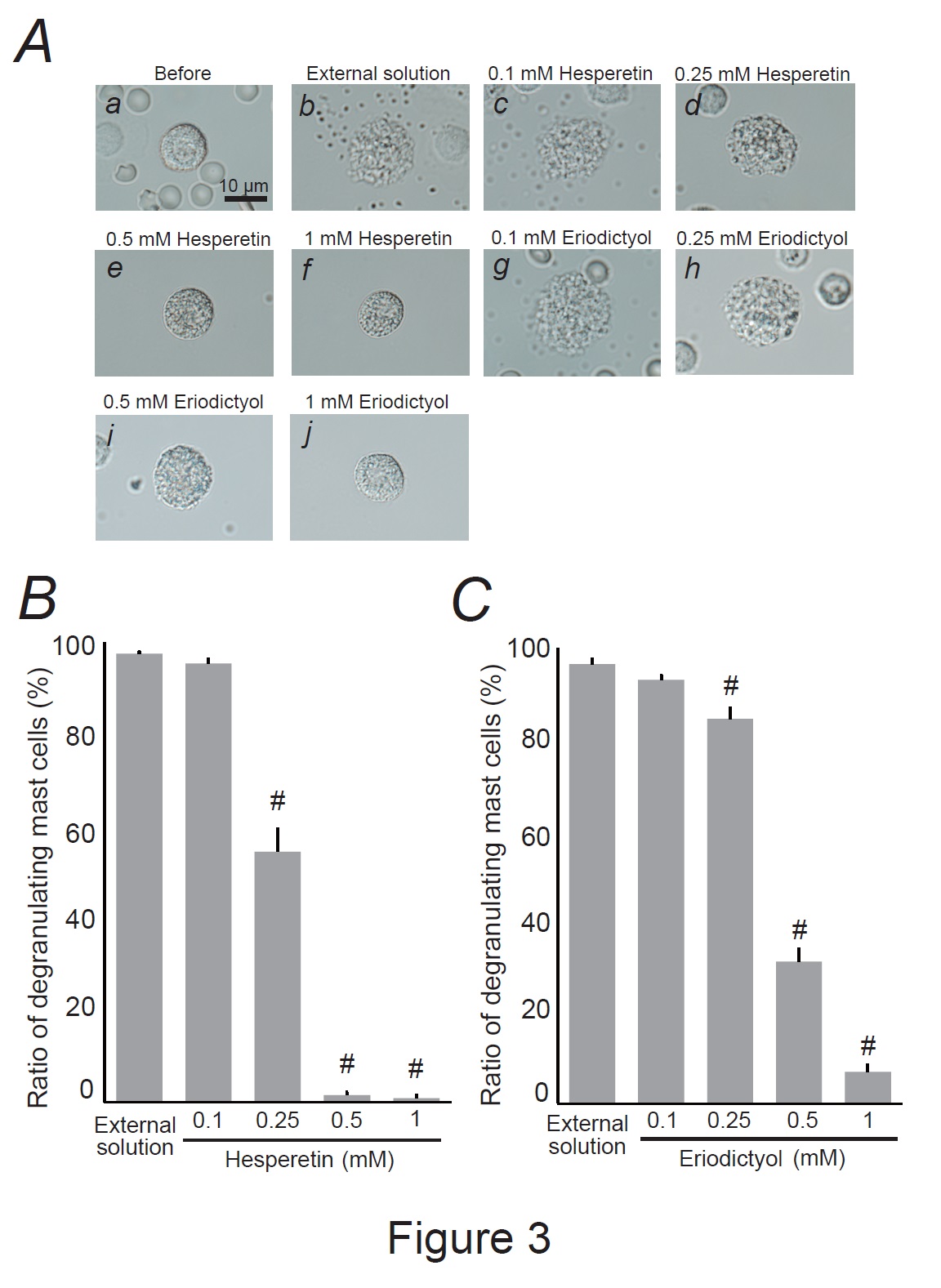
Fig. 3: Effects of hesperetin and eriodictyol on mast cell degranulation. A: Differential-interference contrast (DIC) microscopic images were taken before (a) and after exocytosis was externally induced by compound 48/80 in mast cells incubated in the external solutions containing no substances (b), 0.1 mM hesperetin (c), 0.25 mM hesperetin (d), 0.5 mM hesperetin (e), 1 mM hesperetin (f), 0.1 mM eriodictyol (g), 0.25 mM eriodictyol (h), 0.5 mM eriodictyol (i) and 1 mM eriodictyol (j). Effects of different concentrations (0.1, 0.25, 0.5, and 1 mM) of hesperetin (B) and eriodictyol (C). After the mast cells were incubated in the external solutions containing no substances or either substance, exocytosis was induced by compound 48/80. From a single rat, several samples of mast cell suspension were obtained from the peritoneal cavity. The aliquot of the sample was spread in a chamber placed at the head stage of an inverted microscope. Then bright-field images were obtained from randomly chosen 0.1-mm2 fields of view, in which 30-40 mast cells were evenly observed per field. The degranulating mast cells were expressed as the average percentages of the total mast cells in the 10 bright fields. # p<0.05 vs. incubation in the external solution alone. Values were presented as the means ± SEM. Differences were analyzed by ANOVA followed by Dunnett’s t-test. The experiments were repeated at least three times using three different rats to confirm the reproducibility of the data.
Comparison of mast cell-stabilizing properties among lemon constituents
Our results demonstrated that ascorbic acid required approximately 10 times higher doses than citric acid, hesperetin, and eriodictyol to exert a comparative mast cell-stabilizing effect (Fig. 2 and 3). To clarify the difference in potency, we compared the effects of these constituents at 1 mM (Fig. 4A and B). Similar to the findings for the external solution alone, 1 mM ascorbic acid did not affect exocytosis (Fig. 4Ad vs. b) or the number of degranulating mast cells (Fig. 4B). In contrast, in mast cells incubated with 1 mM citric acid, hesperetin, or eriodictyol, exocytosis was almost completely absent (Fig. 4Ac, e, f) and the number of degranulated mast cells was almost entirely lost (Fig. 4B). These results indicated that citric acid, hesperetin, and eriodictyol are highly potent mast cell-stabilizers (Fig. 5A), and are much more potent than ascorbic acid. Among these constituents, citric acid is by far the most abundant in lemon juice [43] and is considered to be primarily responsible for the anti-allergic properties of lemon [4-6].
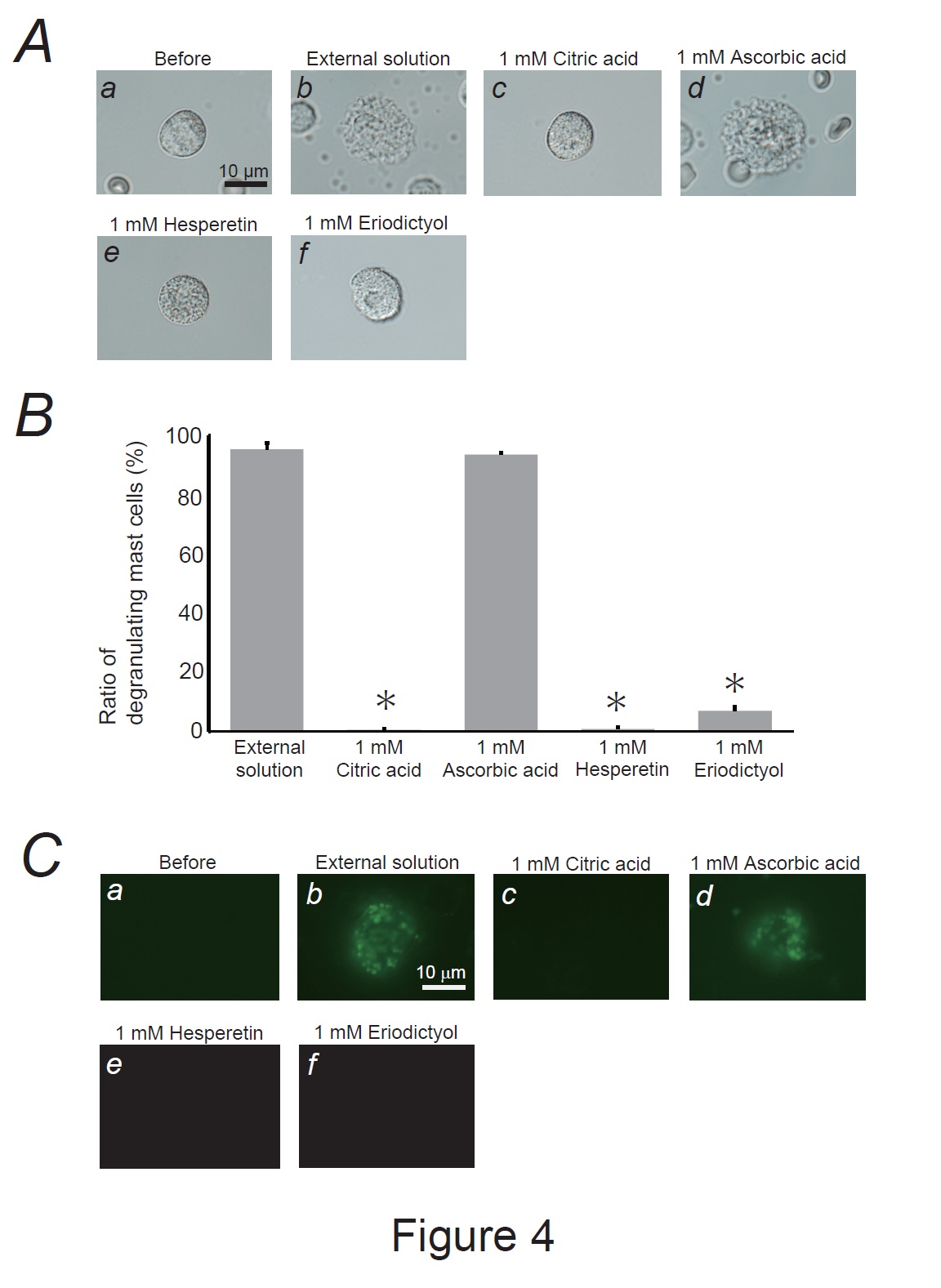
Fig. 4: Effects of lemon constituents on mast cell degranulation and membrane surface deformation due to exocytosis. A: Differential-interference contrast (DIC) microscopic images were taken before (a) and after exocytosis was externally induced by compound 48/80 in mast cells incubated in the external solutions containing no substances (b), 1 mM citric acid (c), ascorbic acid (d), hesperetin (e) and eriodictyol. B: After the mast cells were incubated with the external solutions containing no substances or 1mM citric acid, ascorbic acid, hesperetin and eriodictyol, exocytosis was induced by compound 48/80. From a single rat, several samples of mast cell suspension were obtained from the peritoneal cavity. The aliquot of the sample was spread in a chamber placed at the head stage of an inverted microscope. Then bright-field images were obtained from randomly chosen 0.1-mm2 fields of view, in which 30-40 mast cells were evenly observed per field. The degranulating mast cells were expressed as the average percentages of the total mast cells in the 10 bright fields. *p<0.05 vs. incubation with the external solution containing 1 mM ascorbic acid. Values were presented as the means ± SEM. Differences were analyzed by ANOVA followed by Dunnett’s t-test. The experiments were repeated at least three times using three different rats to confirm the reproducibility of the data. C: The trapping of the fluorescent dye (lucifer yellow) on the cell surface was detected before (a) and after exocytosis was externally induced in mast cells incubated in the external solutions containing no substances (b), 1 mM citric acid (c), ascorbic acid (d), hesperetin (e) and eriodictyol (f).
Effects of lemon constituents on exocytosis-induced membrane surface deformation
According to our previous studies, lipophilic or amphiphilic drugs modulated the process of exocytosis by changing the curvature of the plasma membrane in rat peritoneal mast cells [15, 16, 18, 20, 29]. In the present study, as 1 mM citric acid, hesperetin, and eriodictyol almost completely inhibited the degranulation of mast cells (Fig. 4A and B), these substance-induced changes in membrane architecture would greatly influence the exocytotic process. To examine whether the wrinkles generated in the degranulating mast cells reflected the membrane surface deformation due to exocytosis, we used Lucifer Yellow (Fig. 4C), a water-soluble fluorescent dye retained in the invaginated folds created in the plasma membranes [15, 18, 20, 41, 42]. In mast cells incubated with the external solution alone or with 1 mM ascorbic acid, Lucifer Yellow was trapped almost entirely on the cell surface (Fig. 4Cb, d). Because this membrane-impermeable dye [46] was barely observed in the cells before exocytosis was induced (Fig. 4Ca), positive staining suggested retention of the dye in the opened pores formed by exocytosis [15, 16, 18, 20, 29, 47]. However, after incubating the mast cells with 1mM citric acid, hesperetin, or eriodictyol (Fig. 4Cc, e, and f), the dye was almost completely washed out. Based on these results, citric acid, hesperetin, and eriodictyol inhibited the formation of invaginated folds, counteracting membrane surface deformation due to exocytosis.
Discussion
Besides chemical mediators, such as histamine, serotonin, leukotrienes and prostaglandins, mast cells exocytotically release several cytokines and growth factors [12]. Accordingly, to precisely define the mast cell-stabilizing properties of drugs or substances, exocytosis should be monitored directly rather than indirectly by measuring the amounts of chemical mediators released [15, 16, 18, 48]. Two types of mast cells exist throughout the body [49]. One is the connective tissue type, which primarily exists in loose connective tissues, such as the peritoneal cavity or skin. The other is the mucosal type, which primarily exists in airway or gastrointestinal mucosa. Connective tissue mast cells produce various chemical mediators, such as tryptase, chymase and carboxypeptidases, whereas mucosa type mast cells only produce tryptase [50]. Because the present study focused on the release of whole chemical mediators, we used mast cells isolated from rat peritonei. Additionally, we carefully monitored the entire process of exocytosis under a microscope and defined it as the ratio of degranulating mast cells [15-20]. Using this approach, we have provided in vitro evidence that adrenaline, macrolide antibiotics (clarithromycin), corticosteroids (dexamethasone and hydrocortisone), anti-hypertensives (prazosin), and anti-allergic drugs (tranilast, ketotifen, olopatadine, and cetirizine) exert mast cell-stabilizing properties [15-20]. Additionally, we recently demonstrated that food constituents such as caffeine, catechins, and vitamins, stabilize mast cells and that they exert synergistic effects when combined [21, 22]. In this study, using the same approach, we provided direct evidence that lemon juice and peel constituents or their active metabolites, such as citric acid, hesperetin and eriodictyol, dose-dependently inhibited exocytosis, and thus exerted mast cell-stabilizing properties (Fig. 5A).
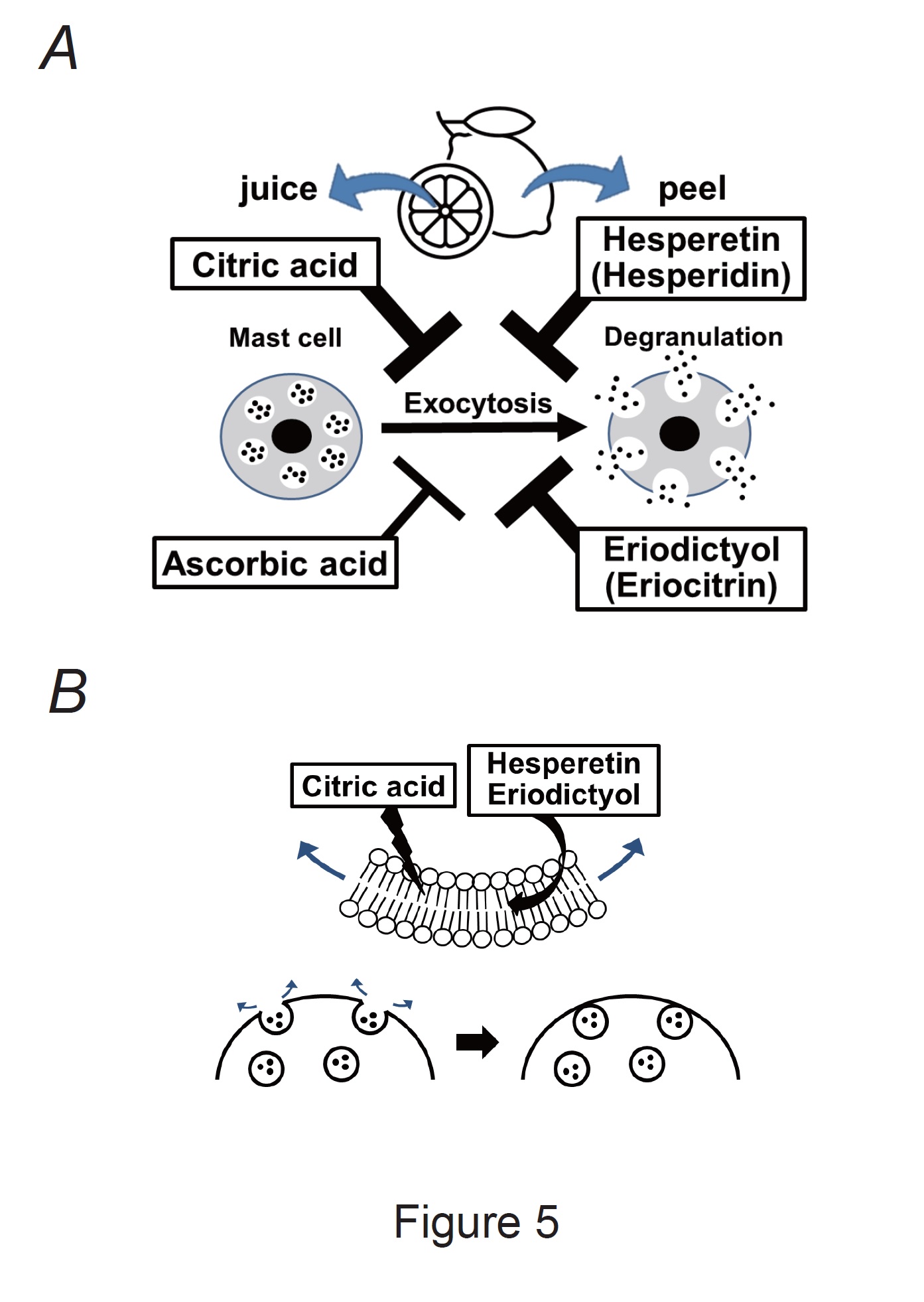
Fig. 5: Mast cell-stabilizing properties of lemon constituents and their proposed mechanisms. A: Citric acid, ascorbic acid, hesperetin, and eriodictyol (active metabolites of hesperidin and eriocitrin) exert mast cell-stabilizing effects. Notably, citric acid, hesperetin, and eriodictyol were more potent than ascorbic acid. B: Citric acid, hesperetin, and eriodictyol directly disturb the plasma membrane of mast cells or interact with lipid bilayers. Thus, they may generate inward membrane bending, thereby counteracting exocytosis in mast cells.
Besides allergic reactions, mast cells contribute to the progression of fibrosis in the lungs, liver, kidneys, and skin [51-53]. Under pathological conditions such as chronic inflammation, mast cells produce fibroblast-activating factors, thus exacerbating organ fibrosis [12]. In this regard, the therapeutic efficacy of mast cell-stabilizers or chemokine inhibitors, which directly suppress mast cell activity, has been demonstrated against organ fibrosis [54-57]. We have previously demonstrated that tranilast, a potent mast cell-stabilizer, reduces the progression of peritoneal fibrosis in a rat model of chronic uremia [16]. In the present study, citric acid, hesperetin, and eriodictyol were found to be highly potent mast cell-stabilizers (Fig. 5A) and may thus be useful in organ fibrosis treatment or prevention. Several recent animal studies have shown that the administration of hesperetin, eriodictyol, and lemon extracts ameliorate organ fibrosis progression, including liver cirrhosis and renal fibrosis [58-61].
In this study, citric acid, hesperetin, and eriodictyol inhibited the formation of invaginated folds and counteracted membrane surface deformation due to exocytosis (Fig. 4C). Because hesperetin and eriodictyol are lipophilic [62], they tend to accumulate inside the plasma membrane and directly interact with its phospholipid bilayers [63, 64] (Fig. 5B). In contrast, at high concentrations, citric acid directly disturbs the plasma membrane structure by changing its integrity and fluidity [65]. In our previous studies, chlorpromazine, which preferentially partitions into the inner leaflet of lipid bilayers, generated inward membrane bending and counteracted exocytosis in mast cells and megakaryocytes [29, 42]. Similarly, in this study, the counteracting effects of these lemon constituents on membrane surface deformation may be attributed to their mast cell-stabilizing properties (Fig. 5B).
In our previous patch-clamp study, the internal application of ethylene glycol tetra-acetic acid (EGTA), which chelates calcium ions and thus inhibits their intracellular transport, completely halted the process of exocytosis [16]. Therefore, consistent with previous findings [66, 67], a rise in intracellular Ca2++ concentrations was considered the primary trigger of mast cell exocytosis. In the present study, we did not examine the dynamics of intracellular Ca2++ concentration after stimulation with the lemon-derived constituents. However, since the constituents, such as eriodictyol, hesperidin, and ascorbic acid, directly suppress the Ca2++ influx in various types of cells [68-70], they would also inhibit Ca2++ signaling in mast cells, thereby exerting additional mast cell-stabilizing effects. Along with transient receptor potential canonical (TRPC) channels that are stimulated by the depletion of Ca2++ stores in the endoplasmic reticulum [71], mast cells express transient potential vanilloid 1 receptor (TRPV1) on the plasma membrane [72]. As TRPV1 is calcium-permeable and allows Ca2++ entry into cells, it contributes to intracellular Ca2++ accumulation in mast cells [73]. In previous study, eriodictyol was shown to antagonize TRPV1 and inhibit Ca2++ influx in spinal cord synaptosomes [72]. Therefore, by antagonizing TRPV1 expressed in mast cells, eriodictyol may exert additional mast cell-stabilizing properties.
Conclusion
This study provides novel in vitro evidence for the first time that lemon juice and peel constituents or their active metabolites, such as citric acid, hesperetin, and eriodictyol, potently exert mast cell-stabilizing properties. These properties are attributable to their inhibitory effects on plasma membrane deformation in degranulating mast cells.
Acknowledgements
Not applicable.
Author contributions
AS and YK performed the experiments and analyzed the data. IK designed the experiments, interpreted the results, and wrote the manuscript. All the authors have read and approved the final version of the manuscript.
Funding
This study was supported by the Tojuro Iijima Foundation for Food Science and Technology, No. 2023-46, Miyagi Kidney Foundation Grant, and the Salt Science Research Foundation, No. 2218, to IK.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics approval and consent to participate
This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals of Miyagi University, which included ethical considerations.
Disclosure Statement
The authors have no competing interests to declare.
References
| 1 | Al-Ataby IA, Talib WH: Daily Consumption of Lemon and Ginger Herbal Infusion Caused Tumor Regression and Activation of the Immune System in a Mouse Model of Breast Cancer. Front Nutr 2022;9:829101.
https://doi.org/10.3389/fnut.2022.829101 |
| 2 | Singh N, Yarla NS, Siddiqi NJ, de Lourdes Pereira M, Sharma B: Features, Pharmacological Chemistry, Molecular Mechanism and Health Benefits of Lemon. Med Chem 2021;17:187-202.
https://doi.org/10.2174/1573406416666200909104050 |
| 3 | Bekkouch O, Zengin G, Harnafi M, Touiss I, Khoulati A, Saalaoui E, Harnafi H, Abdellattif MH, Amrani S: Anti-Inflammatory Study and Phytochemical Characterization of Zingiber officinale Roscoe and Citrus limon L. Juices and Their Formulation. ACS Omega 2023;8:26715-26724.
https://doi.org/10.1021/acsomega.2c04263 |
| 4 | Vazouras KG, Partheniou J, Dimoliatis ID: Alleviation and prevention of severe allergic rhinitis and conjunctivitis following long-term lemon juice use: a case report. Cases J 2009;2:8971.
https://doi.org/10.4076/1757-1626-2-8971 |
| 5 | Grundemann C, Papagiannopoulos M, Lamy E, Mersch-Sundermann V, Huber R: Immunomodulatory properties of a lemon-quince preparation (Gencydo(R)) as an indicator of anti-allergic potency. Phytomedicine 2011;18:760-768.
https://doi.org/10.1016/j.phymed.2010.11.016 |
| 6 | Hoffmann A, Klein SD, Grundemann C, Garcia-Kaufer M, Wolf U, Huber R: Efficacy of a Nasal Spray from Citrus limon and Cydonia oblonga for the Treatment of Hay Fever Symptoms-A Randomized, Placebo Controlled Cross-Over Study. Phytother Res 2016;30:1481-1486.
https://doi.org/10.1002/ptr.5649 |
| 7 | Gonzalez-Molina E, Dominguez-Perles R, Moreno DA, Garcia-Viguera C: Natural bioactive compounds of Citrus limon for food and health. J Pharm Biomed Anal 2010;51:327-345.
https://doi.org/10.1016/j.jpba.2009.07.027 |
| 8 | Avila-Galvez MA, Gimenez-Bastida JA, Gonzalez-Sarrias A, Espin JC: New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study. Antioxidants (Basel) 2021;10
https://doi.org/10.3390/antiox10030435 |
| 9 | Lee HJ, Yang NW, Choi JY, Lee JB, Lee SC: CSP0510 Lotion as a Novel Moisturizer Containing Citric Acid and Trisodium Phosphate Relieves Objective and Subjective Symptoms of Atopic Dermatitis. Ann Dermatol 2016;28:344-351.
https://doi.org/10.5021/ad.2016.28.3.344 |
| 10 | Zhao T, Hu S, Ma P, Che D, Liu R, Zhang Y, Wang J, Li C, Ding Y, Fu J, An H, Gao Z, Zhang T: Neohesperidin suppresses IgE-mediated anaphylactic reactions and mast cell activation via Lyn-PLC-Ca(2+) pathway. Phytother Res 2019;33:2034-2043.
https://doi.org/10.1002/ptr.6385 |
| 11 | Park SJ, Lee YH, Lee KH, Kim TJ: Effect of eriodictyol on the development of atopic dermatitis-like lesions in ICR mice. Biol Pharm Bull 2013;36:1375-1379.
https://doi.org/10.1248/bpb.b13-00296 |
| 12 | Gruber BL: Mast cells in the pathogenesis of fibrosis. Curr Rheumatol Rep 2003;5:147-153.
https://doi.org/10.1007/s11926-003-0043-3 |
| 13 | Church DS, Church MK: Pharmacology of antihistamines. World Allergy Organ J 2011;4:S22-27.
https://doi.org/10.1186/1939-4551-4-S3-S22 |
| 14 | Finn DF, Walsh JJ: Twenty-first century mast cell stabilizers. Br J Pharmacol 2013;170:23-37.
https://doi.org/10.1111/bph.12138 |
| 15 | Baba A, Tachi M, Maruyama Y, Kazama I: Olopatadine inhibits exocytosis in rat peritoneal mast cells by counteracting membrane surface deformation. Cell Physiol Biochem 2015;35:386-396.
https://doi.org/10.1159/000369704 |
| 16 | Baba A, Tachi M, Ejima Y, Endo Y, Toyama H, Matsubara M, Saito K, Yamauchi M, Miura C, Kazama I: Anti-Allergic Drugs Tranilast and Ketotifen Dose-dependently Exert Mast Cell-Stabilizing Properties. Cell Physiol Biochem 2016;38:15-27.
https://doi.org/10.1159/000438605 |
| 17 | Mori T, Abe N, Saito K, Toyama H, Endo Y, Ejima Y, Yamauchi M, Goto M, Mushiake H, Kazama I: Hydrocortisone and dexamethasone dose-dependently stabilize mast cells derived from rat peritoneum. Pharmacol Rep 2016;68:1358-1365.
https://doi.org/10.1016/j.pharep.2016.09.005 |
| 18 | Kazama I, Saito K, Baba A, Mori T, Abe N, Endo Y, Toyama H, Ejima Y, Matsubara M, Yamauchi M: Clarithromycin Dose-Dependently Stabilizes Rat Peritoneal Mast Cells. Chemotherapy 2016;61:295-303.
https://doi.org/10.1159/000445023 |
| 19 | Abe N, Toyama H, Ejima Y, Saito K, Tamada T, Yamauchi M, Kazama I: Prazosin Potentiates Mast Cell-Stabilizing Property of Adrenaline. Cell Physiol Biochem 2024;58:212-225.
https://doi.org/10.33594/000000703 |
| 20 | Fujimura R, Asada A, Aizawa M, Kazama I: Cetirizine more potently exerts mast cell-stabilizing property than diphenhydramine. Drug Discov Ther 2022;16:245-250.
https://doi.org/10.5582/ddt.2022.01067 |
| 21 | Yashima M, Sato Y, Kazama I: Catechin synergistically potentiates mast cell-stabilizing property of caffeine. Allergy Asthma Clin Immunol 2021;17:1.
https://doi.org/10.1186/s13223-020-00502-5 |
| 22 | Kazama I, Sato Y, Tamada T: Pyridoxine Synergistically Potentiates Mast Cell-Stabilizing Property of Ascorbic Acid. Cell Physiol Biochem 2022;56:282-292.
https://doi.org/10.33594/000000534 |
| 23 | Sudakov SK, Alekseeva EV, Nazarova GA, Bashkatova VG: Age-Related Individual Behavioural Characteristics of Adult Wistar Rats. Animals (Basel) 2021;11
https://doi.org/10.20944/preprints202106.0729.v1 |
| 24 | Magro AM, Brai M: Evidence for lipoxygenase activity in induction of histamine release from rat peritoneal mast cells by chelated iron. Immunology 1983;49:1-8.
|
| 25 | Tasaka K, Endo K, Yamasaki H: Degranulation and histamine release in focal antigen-antibody reaction by means of microelectrophoresis in a single rat mesentery mast cell. Jpn J Pharmacol 1972;22:89-95.
https://doi.org/10.1254/jjp.22.89 |
| 26 | Zelechowska P, Agier J, Rozalska S, Wiktorska M, Brzezinska-Blaszczyk E: Leptin stimulates tissue rat mast cell pro-inflammatory activity and migratory response. Inflamm Res 2018;67:789-799.
https://doi.org/10.1007/s00011-018-1171-6 |
| 27 | Mackey E, Moeser AJ: Sex Differences in Mast Cell-Associated Disorders: A Life Span Perspective. Cold Spring Harb Perspect Biol 2022;14
https://doi.org/10.1101/cshperspect.a039172 |
| 28 | Clarkson JM, Martin JE, McKeegan DEF: A review of methods used to kill laboratory rodents: issues and opportunities. Lab Anim 2022;56:419-436.
https://doi.org/10.1177/00236772221097472 |
| 29 | Kazama I, Maruyama Y, Takahashi S, Kokumai T: Amphipaths differentially modulate membrane surface deformation in rat peritoneal mast cells during exocytosis. Cell Physiol Biochem 2013;31:592-600.
https://doi.org/10.1159/000350079 |
| 30 | Caulfield JP, Lewis RA, Hein A, Austen KF: Secretion in dissociated human pulmonary mast cells. Evidence for solubilization of granule contents before discharge. J Cell Biol 1980;85:299-312.
https://doi.org/10.1083/jcb.85.2.299 |
| 31 | Ye J, Piao H, Jiang J, Jin G, Zheng M, Yang J, Jin X, Sun T, Choi YH, Li L, Yan G: Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-kappaB and Nrf2/HO-1 pathways. Sci Rep 2017;7:11895.
https://doi.org/10.1038/s41598-017-12252-3 |
| 32 | Costello LC, Franklin RB: Plasma Citrate Homeostasis: How It Is Regulated; And Its Physiological and Clinical Implications. An Important, But Neglected, Relationship in Medicine. HSOA J Hum Endocrinol 2016;1
https://doi.org/10.24966/HE-9640/100005 |
| 33 | Lykkesfeldt J, Tveden-Nyborg P: The Pharmacokinetics of Vitamin C. Nutrients 2019;11
https://doi.org/10.3390/nu11102412 |
| 34 | Miyake Y, Sakurai C, Usuda M, Fukumoto S, Hiramitsu M, Sakaida K, Osawa T, Kondo K: Difference in plasma metabolite concentration after ingestion of lemon flavonoids and their aglycones in humans. J Nutr Sci Vitaminol (Tokyo) 2006;52:54-60.
https://doi.org/10.3177/jnsv.52.54 |
| 35 | Petillo A, Abruzzese V, Koshal P, Ostuni A, Bisaccia F: Extracellular Citrate Is a Trojan Horse for Cancer Cells. Front Mol Biosci 2020;7:593866.
https://doi.org/10.3389/fmolb.2020.593866 |
| 36 | Cheng Q, Mao L, Huang H, Tang L, Jiang H, Zhang Y, Mu Q: Hesperetin ameliorates glioblastoma by inhibiting proliferation, inducing apoptosis, and suppressing metastasis. Transl Cancer Res 2022;11:1781-1794.
https://doi.org/10.21037/tcr-22-1497 |
| 37 | Shan H, Zhang X, Mi Y, Jia J, Wang B, Yang Q: Eriodictyol Suppresses Gastric Cancer Cells via Inhibition of PI3K/AKT Pathway. Pharmaceuticals (Basel) 2022;15
https://doi.org/10.3390/ph15121477 |
| 38 | Mojic M, Bogdanovic Pristov J, Maksimovic-Ivanic D, Jones DR, Stanic M, Mijatovic S, Spasojevic I: Extracellular iron diminishes anticancer effects of vitamin C: an in vitro study. Sci Rep 2014;4:5955.
https://doi.org/10.1038/srep05955 |
| 39 | Hosokawa Y, Saga R, Monzen S, Terashima S, Tsuruga E: Ascorbic acid does not reduce the anticancer effect of radiotherapy. Biomed Rep 2017;6:103-107.
https://doi.org/10.3892/br.2016.819 |
| 40 | Barrett KE, Pearce FL: A comparison of histamine secretion from isolated peritoneal mast cells of the mouse and rat. Int Arch Allergy Appl Immunol 1983;72:234-238.
https://doi.org/10.1159/000234873 |
| 41 | Odriscoll D, Wilson G, Steer MW: Lucifer Yellow and Fluorescein Isothiocyanate Uptake by Cells of Morinda-Citrifolia in Suspension-Cultures Is Not Confined to the Endocytotic Pathway. J Cell Sci 1991;100:237-241.
https://doi.org/10.1242/jcs.100.1.237 |
| 42 | Kazama I, Ejima Y, Endo Y, Toyama H, Matsubara M, Baba A, Tachi M: Chlorpromazine-induced changes in membrane micro-architecture inhibit thrombopoiesis in rat megakaryocytes. Biochim Biophys Acta 2015;1848:2805-2812.
https://doi.org/10.1016/j.bbamem.2015.08.013 |
| 43 | Penniston KL, Nakada SY, Holmes RP, Assimos DG: Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J Endourol 2008;22:567-570.
https://doi.org/10.1089/end.2007.0304 |
| 44 | Miyake Y, Yamamoto K, Tsujihara N, Osawa T: Protective effects of lemon flavonoids on oxidative stress in diabetic rats. Lipids 1998;33:689-695.
https://doi.org/10.1007/s11745-998-0258-y |
| 45 | Williamson G, Kay CD, Crozier A: The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr Rev Food Sci Food Saf 2018;17:1054-1112.
https://doi.org/10.1111/1541-4337.12351 |
| 46 | LaPlaca MC, Prado GR, Cullen D, Simon CM: Plasma membrane damage as a marker of neuronal injury. Conf Proc IEEE Eng Med Biol Soc 2009;2009:1113-1116.
https://doi.org/10.1109/IEMBS.2009.5334457 |
| 47 | Kawasaki Y, Saitoh T, Okabe T, Kumakura K, Ohara-Imaizumi M: Visualization of exocytotic secretory processes of mast cells by fluorescence techniques. Biochim Biophys Acta 1991;1067:71-80.
https://doi.org/10.1016/0005-2736(91)90027-6 |
| 48 | Yoo JM, Kim JH, Park SJ, Kang YJ, Kim TJ: Inhibitory effect of eriodictyol on IgE/Ag-induced type I hypersensitivity. Biosci Biotechnol Biochem 2012;76:1285-1290.
https://doi.org/10.1271/bbb.110952 |
| 49 | Wasserman SI: Mast cell biology. J Allergy Clin Immunol 1990;86:590-593.
https://doi.org/10.1016/S0091-6749(05)80221-0 |
| 50 | Krystel-Whittemore M, Dileepan KN, Wood JG: Mast Cell: A Multi-Functional Master Cell. Front Immunol 2015;6:620.
https://doi.org/10.3389/fimmu.2015.00620 |
| 51 | Gruber BL: Mast cells: accessory cells which potentiate fibrosis. Int Rev Immunol 1995;12:259-279.
https://doi.org/10.3109/08830189509056717 |
| 52 | Holdsworth SR, Summers SA: Role of mast cells in progressive renal diseases. J Am Soc Nephrol 2008;19:2254-2261.
https://doi.org/10.1681/ASN.2008010015 |
| 53 | Blank U, Essig M, Scandiuzzi L, Benhamou M, Kanamaru Y: Mast cells and inflammatory kidney disease. Immunol Rev 2007;217:79-95.
https://doi.org/10.1111/j.1600-065X.2007.00503.x |
| 54 | Miyajima A, Asano T, Yoshimura I, Seta K, Hayakawa M: Tranilast ameliorates renal tubular damage in unilateral ureteral obstruction. J Urol 2001;165:1714-1718.
https://doi.org/10.1016/S0022-5347(05)66400-2 |
| 55 | Kelly DJ, Zhang Y, Gow R, Gilbert RE: Tranilast attenuates structural and functional aspects of renal injury in the remnant kidney model. J Am Soc Nephrol 2004;15:2619-2629.
https://doi.org/10.1097/01.ASN.0000139066.77892.04 |
| 56 | Shiota N, Kakizoe E, Shimoura K, Tanaka T, Okunishi H: Effect of mast cell chymase inhibitor on the development of scleroderma in tight-skin mice. Br J Pharmacol 2005;145:424-431.
https://doi.org/10.1038/sj.bjp.0706209 |
| 57 | Doggrell SA, Wanstall JC: Cardiac chymase: pathophysiological role and therapeutic potential of chymase inhibitors. Can J Physiol Pharmacol 2005;83:123-130.
https://doi.org/10.1139/y04-136 |
| 58 | Kong R, Wang N, Luo H, Lu J: Hesperetin Mitigates Bile Duct Ligation-Induced Liver Fibrosis by Inhibiting Extracellular Matrix and Cell Apoptosis via the TGF-beta1/Smad Pathway. Curr Mol Med 2018;18:15-24.
https://doi.org/10.2174/1566524018666180608084947 |
| 59 | Li JJ, Jiang HC, Wang A, Bu FT, Jia PC, Zhu S, Zhu L, Huang C, Li J: Hesperetin derivative-16 attenuates CCl(4)-induced inflammation and liver fibrosis by activating AMPK/SIRT3 pathway. Eur J Pharmacol 2022;915:174530.
https://doi.org/10.1016/j.ejphar.2021.174530 |
| 60 | AlTamimi JZ, AlFaris NA, Alshammari GM, Alagal RI, Aljabryn DH, Abdo Yahya M: Protective effect of eriodictyol against hyperglycemia-induced diabetic nephropathy in rats entails antioxidant and anti-inflammatory effects mediated by activating Nrf2. Saudi Pharm J 2023;31:101817.
https://doi.org/10.1016/j.jsps.2023.101817 |
| 61 | Chen YJL, Chou PC, Hsu CL, Hung JF, Wu YC, Lin JG: Fermented Citrus Lemon Reduces Liver Injury Induced by Carbon Tetrachloride in Rats. Evid Based Complement Alternat Med 2018;2018:6546808.
https://doi.org/10.1155/2018/6546808 |
| 62 | Shubina VS, Kozina VI, Shatalin YV: A Comparative Study of the Inhibitory Effect of Some Flavonoids and a Conjugate of Taxifolin with Glyoxylic Acid on the Oxidative Burst of Neutrophils. Int J Mol Sci 2023;24
https://doi.org/10.3390/ijms242015068 |
| 63 | Londono-Londono J, Lima VR, Jaramillo C, Creczynski-Pasa T: Hesperidin and hesperetin membrane interaction: understanding the role of 7-O-glycoside moiety in flavonoids. Arch Biochem Biophys 2010;499:6-16.
https://doi.org/10.1016/j.abb.2010.04.023 |
| 64 | Choi SS, Lee SH, Lee KA: A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In vitro. Antioxidants (Basel) 2022;11
https://doi.org/10.3390/antiox11081618 |
| 65 | Meng X, Liu X, Bao Y, Luo T, Wang J: Effect of citric acid on cell membrane structure and function of Issatchenkia terricola WJL-G4. J Appl Microbiol 2024;135
https://doi.org/10.1093/jambio/lxae057 |
| 66 | Oshiro T, Kakuta Y, Maruyama N, Fushimi T, Okayama H, Tamura G, Shimura S, Shirato K: Patch-clamp characterization of secretory process in human basophils. Int Arch Allergy Immunol 1997;112:336-340.
https://doi.org/10.1159/000237477 |
| 67 | Neher E: The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol 1988;395:193-214.
https://doi.org/10.1113/jphysiol.1988.sp016914 |
| 68 | Lee M, Shim SY: Inhibitory Effects of Eriodictyol-7-O-beta-d-glucuronide and 5, 7-Dihydroxy-4-chromene Isolated from Chrysanthemum zawadskii var. latilobum in FcepsilonRI-Mediated Human Basophilic KU812F Cell Activation. Molecules 2020;25
https://doi.org/10.3390/molecules25040994 |
| 69 | Maekawa S, Sato K, Fujita K, Daigaku R, Tawarayama H, Murayama N, Moritoh S, Yabana T, Shiga Y, Omodaka K, Maruyama K, Nishiguchi KM, Nakazawa T: The neuroprotective effect of hesperidin in NMDA-induced retinal injury acts by suppressing oxidative stress and excessive calpain activation. Sci Rep 2017;7:6885.
https://doi.org/10.1038/s41598-017-06969-4 |
| 70 | Ozturk G, Mulholland CW, Hannigan BM: Vitamin C decreases intracellular calcium level in human lymphoid cells. J Physiol Pharmacol 2001;52:285-292.
|
| 71 | Ashmole I, Bradding P: Ion channels regulating mast cell biology. Clin Exp Allergy 2013;43:491-502.
https://doi.org/10.1111/cea.12043 |
| 72 | Rossato MF, Trevisan G, Walker CI, Klafke JZ, de Oliveira AP, Villarinho JG, Zanon RB, Royes LF, Athayde ML, Gomez MV, Ferreira J: Eriodictyol: a flavonoid antagonist of the TRPV1 receptor with antioxidant activity. Biochem Pharmacol 2011;81:544-551.
https://doi.org/10.1016/j.bcp.2010.11.004 |
| 73 | Solis-Lopez A, Kriebs U, Marx A, Mannebach S, Liedtke WB, Caterina MJ, Freichel M, Tsvilovskyy VV: Analysis of TRPV channel activation by stimulation of FCepsilonRI and MRGPR receptors in mouse peritoneal mast cells. PLoS One 2017;12:e0171366.
https://doi.org/10.1371/journal.pone.0171366 |