Original Article - DOI:10.33594/000000744
Accepted 13 November 2024 - Published online 28 November 2024
Therapeutic Effect of Mesenchymal Stem Cells and Olive Leaf Extract Against Cytotoxicity Induced by Streptozotocin in Gestational Diabetes Rats
Keywords
Abstract
Background/Aims:
Gestational Diabetes Mellitus (GDM), a prevalent complication in pregnancy, is characterized by the Diabetes Association as diabetes diagnosed in the second or third trimester, often remaining asymptomatic. This study investigates the intricate effects of Streptozotocin on pregnant rats, unraveling its impact on Gestational Type 2 Diabetes (GTD). The research delves into the potential therapeutic roles of mesenchymal stem cells (MSCs) and olive leaf extract (OLE) in mitigating the consequences of Streptozotocin-induced pancreatic impairment.Methods:
Female rats were divided into control group, diabetic group, group treated with stem cells after diabetes, group treated with olive leaf extract after diabetes, and group treated with olive leaf extract and stem cells after diabetes. A comprehensive analysis was conducted for all study groups, including diabetic patients’ files, hormonal dynamics, histopathological changes, tissue immune responses, and antioxidant mechanisms for all study groups.Results:
The results of the study showed that diabetes causes changes in the level of insulin, HB A1C, Fructosamine, HOMA-IR and hormones compared to control group. On the contrary, there was a noticeable improvement in these parameters after treatment with olive leaf extract and stem cells. The results of the present study demonstrated that the combination of OLE and MSCs emerges as a promising approach, demonstrating enhanced glycemic control, modulation of insulin resistance, and improvements in pancreatic islet health. Aso, histopathological results underscore positive effects on pancreatic islet integrity.Conclusion:
Stem Cells and Olive Leaf Extract demonstrate favorable effects on regenerative pancreatic cells under normal conditions.Introduction
Diabetes mellitus (DM) is characterized by a compromised ability to regulate blood glucose levels in both humans and animals. This condition results from either insufficient insulin production or disruptions in signaling pathways [1, 2]. The disturbance in the equilibrium of blood glucose significantly impacts the metabolism of key nutrients, affecting not only carbohydrates but also lipids and proteins [3]. Gestational diabetes mellitus (GDM) is associated with both immediate adverse obstetric and perinatal complications, as well as persistent metabolic health effects on the offspring [4]. This phenomenon is believed to be influenced by fetal programming, a fundamental mechanism linking exposure to maternal diabetes during pregnancy with an increased vulnerability to metabolic issues in adulthood, [5]. The placenta, functioning as a crucial interface connecting the mother and fetus, plays a pivotal role in facilitating fetal growth and development. Simultaneously, it adjusts to the maternal nutritional and metabolic status [6]. In addition to its widely recognized roles in endocrine, immunological, and vascular functions, the placenta is an intricately organized organ consisting of various cell types with specialized functions in regulating nutrient transport and energy metabolism [7]. The influence of maternal glycemic control on placental dysfunction is a subject of ongoing debate. However, distinctive characteristics of Gestational Diabetes Mellitus (GDM) include an augmented size, altered vascular permeability, and the occurrence of mild inflammation [8]. Such disturbances possess the capacity to influence fetal precursor cells located in the placenta and/or umbilical cord, thereby establishing a unique pathway that markedly contributes to the onset of metabolic disorders in the offspring of mothers affected by gestational diabetes mellitus (GDM)[9]. Recent studies have elucidated the significant impact of gestational diabetes mellitus (GDM) on bone marrow-derived stromal cells, revealing effects on proliferation, mitochondrial function, and angiogenesis [10]. Streptozotocin (STZ), initially isolated from Streptomyces achromogenes in 1960, had its diabetogenic properties unveiled in 1963[11]. This action was characterized by Junod, Lambert, Stauffacher, and Renold in 1969 [12] building upon earlier research from 1967[13], which demonstrated that the selective destruction of pancreatic islet β-cells underlies the diabetogenic effects. Consequently, this process leads to insulin deficiency, hyperglycemia, polydipsia, and polyuria in animals, mirroring the characteristic symptoms of human type 1 diabetes mellitus [14]. Contemporary therapeutic approaches for diabetes, including Glycemic Target-Directed (GTD) strategies, encompass lifestyle modifications, oral medicinal protocols, injectable insulin administration, and promising interventions such as GLP-1 agonists [15]. Challenges associated with these methods include difficulties in adhering to lifestyle changes, potential adverse effects and inconsistent reactions to medicinal regimens, the risk of insulin-induced hypoglycemia, and the imperative for personalized treatment plans to achieve optimal glycemic regulation [16].
Mesenchymal stem cells (MSCs) are a type of multipotent stem cell known for their remarkable ability to generate precursor cells and differentiate into various lineages within the mesenchymal lineage [17, 18]. The utilization of MSC therapy is emerging as a promising avenue in the field of regenerative medicine, particularly for pancreas rejuvenation and the repair of beta cells and surrounding tissues [19]. The efficacy of MSCs in cell therapy depends on their efficient colonization of damaged or target tissues, cellular viability, and their potential to integrate and persist within the relevant tissue over the long term [20].Numerous investigations have illustrated the potential of MSCs as a cellular-based approach for addressing a spectrum of ailments, including liver damage, diabetes mellitus, neurodegenerative disorders, and burn skin treatment [21-23].These alterations involve diminished expression levels of specific surface markers, abnormal cellular structures, compromised self-renewal ability, and an accelerated proliferation rate leading to cellular senescence. This evolving concern presents a significant obstacle to the effective clinical deployment of MSC-based cellular therapy [7, 24, 25]. Despite the limitations of commercially available anti-diabetic drugs and their associated side effects, attention is shifting towards the exploration of innovative antidiabetic medications [26]. Plant-based therapeutics are generally perceived as less or non-toxic and devoid of adverse effects [27]. Given the cost and adverse effects associated with synthetic drugs, there is a strong focus on identifying novel and effective alternative antidiabetic sources [28]. Olive leaf extract, containing bioactive compounds with potential therapeutic benefits, has been investigated for its applications in wound healing, cardiovascular diseases, and neurodegenerative disorders [29, 30]. In the context of diabetes management, stem cells hold the potential to regenerate pancreatic cells, while the antioxidant and anti-inflammatory properties of olive leaf extract could contribute to glucose control. However, further research is essential for clinical validation of these potential benefits [31].
This research endeavors to address knowledge gaps concerning the impact of interventions involving stem cells and olive leaf extract on gestational type 2 diabetes. The principal objectives center on gaining insights into the effects of these interventions and formulating hypotheses regarding their ability to mitigate symptoms. The study adopts a thorough approach, employing diverse methodologies such as profiling, hormonal analysis, histopathology, immuno-histochemistry, and antioxidant assessment for a comprehensive evaluation.
This study aims to investigate the therapeutic effects of mesenchymal stem cells (MSCs) and olive leaf extract (OLE) in mitigating the cytotoxic effects of Streptozotocin-induced gestational diabetes in rats, focusing on glycemic control, pancreatic islet health, and insulin resistance.
Materials and Methods
Animal
The female albino rats utilized in this study were procured from VACSERA Co., Egypt, and were aged 8-9 weeks, with a weight range of 150-170 g. The rats were housed individually in a controlled environment set at 25°C, with a 12-hour light-dark cycle [32]. Two distinct groups were established: a control group subjected to a 12-hour light-dark cycle and a gestational diabetes mellitus (GDM) group exposed to an 18-hour light-4-hour dark cycle, known to induce oxidative stress [33]. The entire research protocol received ethical approval from the Fayoum University Institutional Animal Care and Use Committee (FU-IACUC) under Code No. AEC 2304. This approval was granted in strict adherence to the Global Strategies for the Ethical Treatment and Utilization of Laboratory Animals. Female albino rats were chosen for this study due to their sensitivity to STZ-induced gestational diabetes and established use in reproductive research. This strain’s physiological responses to STZ provide a reliable model for studying gestational diabetes, allowing for reproducible results in evaluating therapeutic interventions.
Preparation of the Olive Leaf Extract (OLE)
Olive leaf extract (OLE) was derived from olive tree leaves. The resultant dry residue was meticulously weighed, subsequently dissolved in water, and maintained at a temperature of -20°C until application as a therapeutic agent. Olive Leaf Extract (OLE) was administered orally to rats by gavage at a dose of 200 mg/kg body weight once daily. This administration began after the establishment of stable hyperglycemia, as verified by blood glucose measurements 72 hours post-STZ injection.
Isolation and characterization of Bone Marrow Mesenchymal Stem Cells (BM-MSCs)
BM-MSCs were procured from the bone marrow of 4-week-old rats, adhering to established protocols [34]. Briefly, the rats were euthanized, and their femurs and tibiae were collected and meticulously cleaned to eliminate any muscle or connective tissue. The bone marrow was subsequently rinsed and cultured in a low-glucose DMEM solution containing 10% fetal bovine serum (FBS, Invitrogen Australia Pty Ltd., Mount Waverley, Victoria, Australia) and 1% penicillin/streptomycin. Following epiphysis removal, the bone marrow was maintained in a humidified environment with 5% CO2 at 37°C. The culture medium was refreshed biweekly until the cells achieved an approximate 80% confluence. BM-MSCs from the third and fourth passages were utilized in all experiments. The BM-MSCs in their third passage underwent a series of steps involving washing, purification, and targeting specific cell markers, with evaluation conducted using a flow cytometer. To ensure the exclusive examination of viable cells, forward and side scatter measurements were employed to exclude deceased cells and debris. Each sample underwent gating to encompass over 10, 000 events [35].
Experimental Design
The experimental setup involved the utilization of 50 pregnant female rats, subjected to vaginal smear examination to ascertain the phases of the estrous cycle. Rats deviating from the regular 4-day estrous cycle were excluded from the study. Subsequently, the rats were randomly allocated into five groups, each comprising ten rats: 1. Control group: pregnant rats without treatments 2. Gestationally Diabetic Group (GD): Pregnant rats received a single intraperitoneal (ip) injection of Streptozotocin (STZ) at a dose of 35 mg/kg body weight on Day 0 of gestation. Blood glucose levels were assessed 72 hours after STZ administration to identify rats displaying stable hyperglycemia [36]. 3. Gestationally Diabetic Group treated with Mesenchymal Stem Cells (GD + MSCs): Pregnant rats received an injection of (1x106) bone marrow-derived mesenchymal stem cells (BM-MSCs) suspended in 500 µL PBS, administered via caudal vein injection after diabetes occurred. 4. Gestationally Diabetic Group Treated with Olive Leaf Extract (GD + OLE): Pregnant rats received olive leaf extracts (OLE) at a dose of 200 mg extract/Kg body weight after diabetes occurred. 5. Gestationally Diabetic Group Treated with Mesenchymal Stem Cells and Olive Leaf Extract (GD + OLE + MSCs): This group involved the combined treatment of gestationally diabetic rats with both mesenchymal stem cells and olive leaf extract. The criteria for exclusion from the study included any deviations from the 4-day estrous cycle pattern. Furthermore, stable hyperglycemia, a key indicator, was assessed 72 hours post-Streptozotocin administration in the gestationally diabetic group. These well-defined experimental groups provide a robust foundation for investigating the effects of different interventions on gestational diabetes in rats. In the GD + MSCs, GD + OLE, and GD + OLE + MSCs groups, interventions were initiated 72 hours post-STZ injection. At this point, stable hyperglycemia was confirmed through blood glucose testing, signifying the onset of diabetes. MSCs (1x106 cells in 500 µL PBS) were transplanted via caudal vein injection, and OLE was given orally by gavage at a dose of 200 mg/kg body weight daily.
Blood Sample Collection
On Day 21 of gestation, blood samples were procured through the jugular plexus veins and cardiac puncture for subsequent biochemical and hematological analyses. Serum was obtained using standard tubes, while plasma was collected in tubes containing heparin. Coagulation in the standard tubes occurred over a 30-minute period, whereas heparin tubes were refrigerated to arrest the coagulation process. Both tube types underwent centrifugation at 2500 rpm for 12 minutes at -4°C. Following centrifugation, the serum and plasma were carefully transferred to microcentrifuge tubes and preserved at -80°C. Both serum and plasma tubes were centrifuged at 2500 rpm for 12 minutes at +4°C. This correction ensures accurate reporting of sample processing conditions.
Diabetic Profile Assessment
The evaluation of Streptozotocin-induced metabolic disruption included the measurement of HbA1c following the method outlined by Trinder [37], fructosamine as per Armbruster [38], glucose, insulin based on the protocol described by Matthews et al. [39], and HOMA-IR according to the methodology outlined by Genovesi et al. [40].
Hormonal Dynamics
The thyroid-stimulating hormone (TSH), as per the study conducted by Leirs et al. [41], the follicle-stimulating hormone (FSH) and luteinizing hormone (LH), as investigated by Bablok et al. [42], Additionally, prolactin (PRL), as studied by Alving in 1998, and progesterone levels, as examined by Pedersen et al. [43].The study aims to evaluate the interplay between hormonal imbalances and GD, focusing on the influence of stem cells and olive leaf extract on the modulation of TSH, FSH, LH, PRL, and progesterone levels.
Histopathological Assessment
Formalin-preserved pancreatic specimens obtained from rat dams and near full-term embryos underwent automated tissue processing. This process involved a two-step fixation and dehydration procedure. Initially, the fixation step entailed immersing the tissue in a 10% buffered formalin solution for a duration of 48 hours, followed by a 30-minute wash in distilled water. Subsequently, dehydration was carried out through sequential exposure to alcohol at concentrations of 70%, 90%, and 100%. Following specific immersion times in alcohol, the samples underwent clearing in xylene and impregnation with paraffin wax. The resulting paraffin sections, with a thickness of 4-5 micrometers, were stained with hematoxylin and eosin for the purpose of examining circulatory disturbances, inflammation, degenerations, apoptosis, necrosis, and other pathological changes [44].
Immunohistochemistry
The tissue sections underwent microwave treatment and underwent a two-step immunostaining process. The primary antibody was bound to the corresponding antigen, and visualization was achieved using a biotin-streptavidin (BSA) system, as described by Janardhan et al. [45]. Diaminobenzidine (DAB) was employed for permanent staining, while hematoxylin served as the counterstain. Paraffin sections (5 µm) were affixed to glass slides, treated with Xylene and ethanol, and subsequently incubated with primary monoclonal antibodies targeting Insulin, PCNA, and P53. The ensuing procedures involved DAKO EnVision application, DAB chromogen application, counterstaining with hematoxylin, and final mounting, following the protocol outlined by Mok et al. [46].
Statistical analysis
SPSS Statistics 23.0 was employed for data analysis, utilizing descriptive statistics. Paired t-tests were utilized to compare two groups. In the case of multiple groups, ANOVA was employed for assessing normally distributed data, whereas related sample Wilcoxon signed-rank tests were applied for non-normally distributed data [47].
Results
Table 1 and Fig. 1 presents the statistical analysis of HB A1C, serum Fructosamine, glucose, insulin, and HOMA-IR in female albino rats across different treatment groups. The results are expressed as means with standard errors, and significance is denoted by different letters (a, b, c, d, e) within each parameter. The HB A1C levels varied significantly among groups, with the control and the group treated with both olive and stem cell showing lower values compared to diabetic and diabetic + stem cell groups. This suggests a potential ameliorative effect of the combined treatment on HB A1C in diabetic rats. Serum Fructosamine levels followed a similar trend, with the control and the group receiving both olive and stem cell treatments exhibiting lower values compared to diabetic rats. This implies a positive impact of the combined treatment on Fructosamine levels in diabetic conditions. Glucose levels were significantly elevated in diabetic rats compared to the control. However, the diabetic + olive, diabetic + stem cell, and diabetic + olive + stem cell groups demonstrated varying degrees of reduction, indicating potential glycemic control with these treatments. Insulin levels exhibited a significant decrease in diabetic rats compared to the control, while the groups treated with olive, stem cell, and their combination showed an increase, suggesting a potential role in insulin regulation. HOMA-IR, an index of insulin resistance, was significantly elevated in diabetic rats but showed improvements in the groups treated with olive, stem cell, and their combination. This implies a potential attenuation of insulin resistance with these treatments. Overall, the results highlight the potential therapeutic effects of olive, stem cell, and their combination in mitigating diabetes-induced alterations in HB A1C, Fructosamine, glucose, insulin, and HOMA-IR in female albino rats. The utilization of statistical analysis and the clear presentation of results contribute to the scientific rigor of the study.
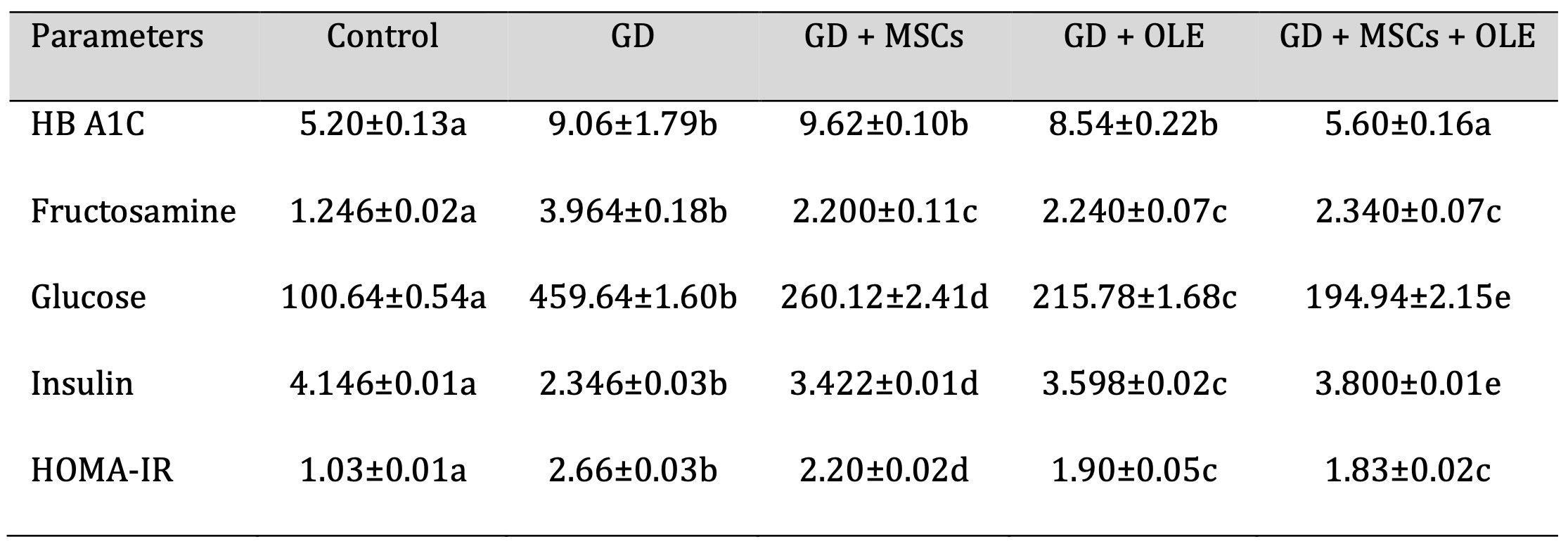
Table 1: Effects of olive leaf extract (OLE), mesenchymal stem cells (MSC) and their combination on elevated glycemic markers of gestational diabetic rats. Each value depicted signifies the mean of five records with a standard error of measurement. The use of identical letters (a, b, c, d,e) to represent groups signifies their lack of statistically significant variance. Conversely, different letters signify a significant variation
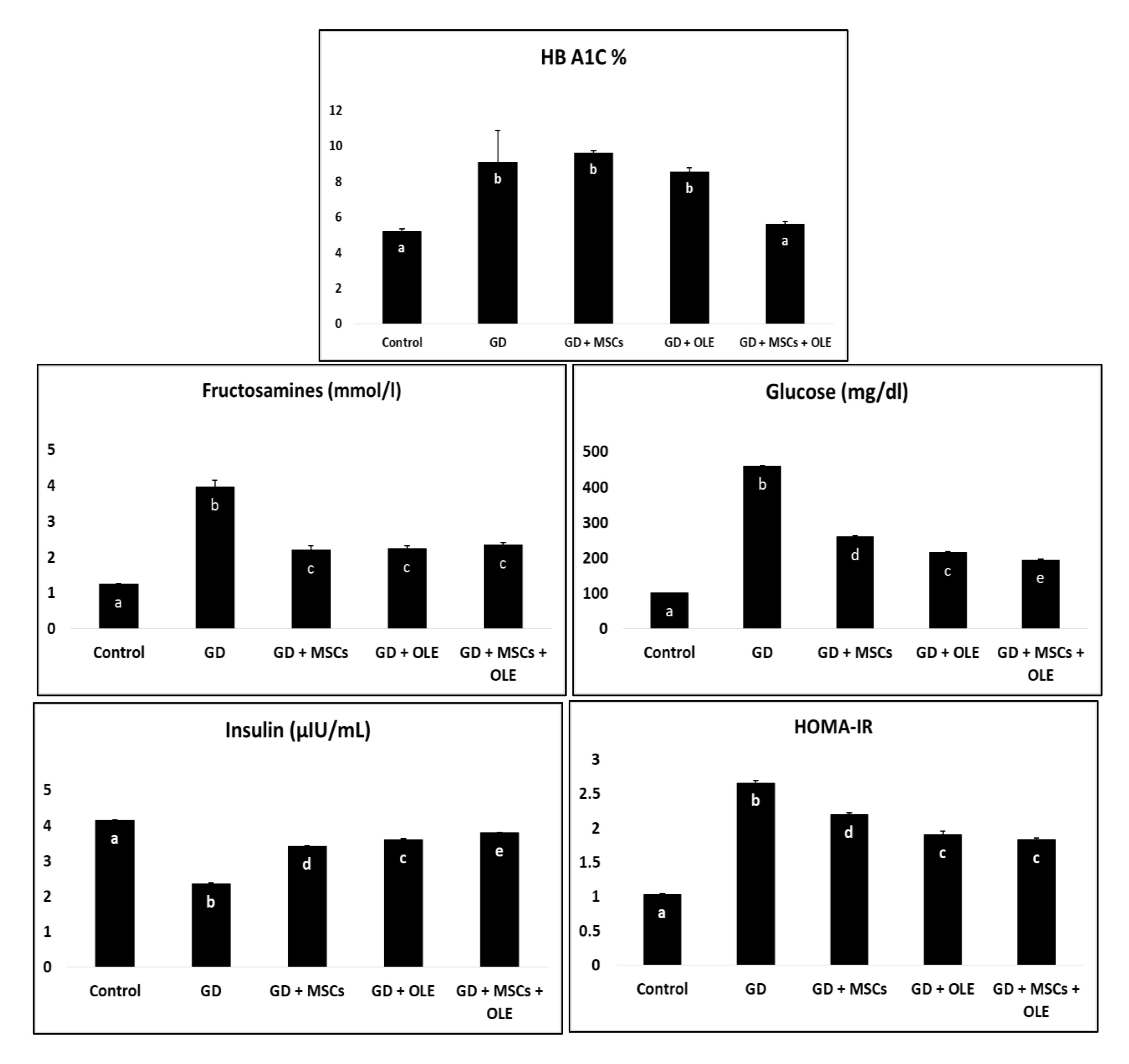
Fig. 1: Effects of olive leaf extract (OLE), mesenchymal stem cells (MSC) and their combination on elevated glycemic markers of gestational diabetic rats.
Table 2 and Fig. 2 presents a comprehensive analysis of various parameters in different experimental groups, namely Control, Diabetic, Diabetic + Olive, Diabetic + Stem Cell, and Diabetic + Olive + Stem Cell. The parameters under consideration include TSH, FSH, LH, PRL, and Progesterone. Statistically, significant differences are observed in TSH levels among the groups, with values ranging from 1.45±0.01 in the Control group to 1.68±0.04 in the Diabetic + Olive + Stem Cell group. Similar trends are noted for FSH, LH, PRL, and Progesterone, with each group exhibiting distinct values. The alphabetical annotations (a, b, c, d, e) provide a clear indication of the significance of differences between groups. Groups sharing the same letter indicate no significant difference, while differing letters signify a notable change. For instance, in TSH levels, the Control group (a) differs significantly from the Diabetic (b), Diabetic + Olive (c), Diabetic + Stem Cell (d), and Diabetic + Olive + Stem Cell (e) groups. Additionally, the percentage values represent the magnitude of changes relative to the Control group, offering a quantifiable perspective on alterations. This analytical approach ensures scientific rigor, allowing for a thorough understanding of the statistical significance and trends in the data (Table 2).
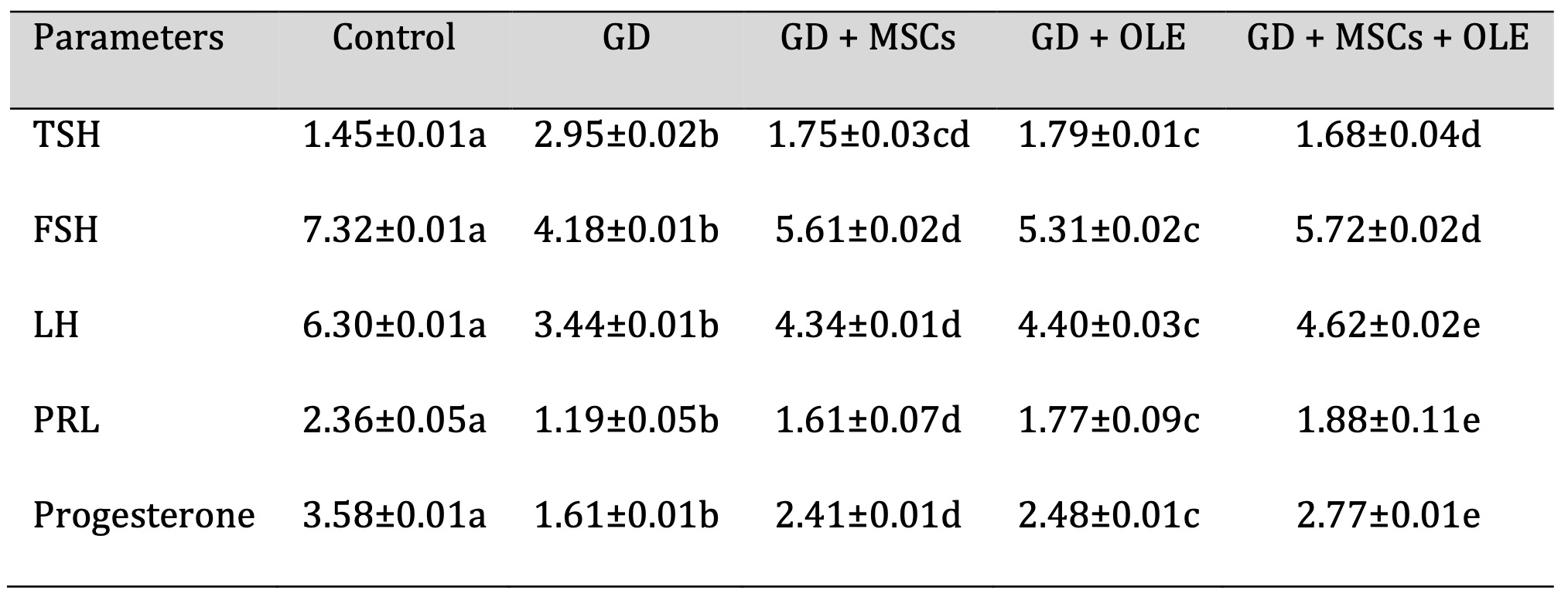
Table 2: Effects of olive leaf extract (OLE), mesenchymal stem cells (MSC) and their combination on elevated hormonal biochemical of gestational diabetic rats. Each value depicted signifies the mean of five records with a standard error of measurement. The use of identical letters (a, b, c, d,e) to represent groups signifies their lack of statistically significant variance. Conversely, different letters signify a significant variation
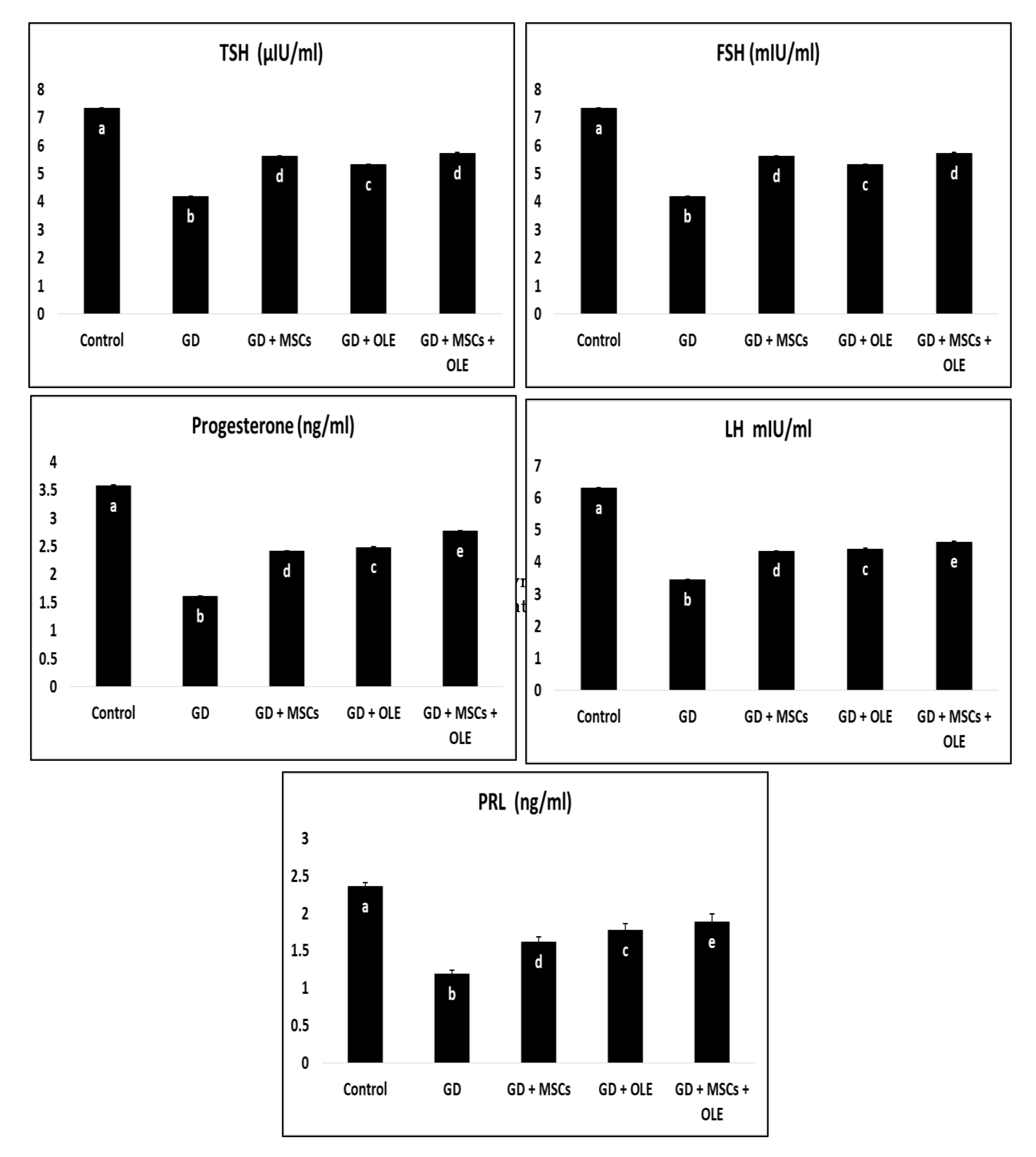
Fig. 2: Effects of olive leaf extract (OLE), mesenchymal stem cells (MSC) and their combination on elevated hormonal biochemical of gestational diabetic rats.
Histopathology
Serial sections of the adult rat’s pancreas in the negative control group (G1) revealed ostensibly normal exocrine and endocrine components, including a healthy acinar epithelium and its secretory granules. The endocrine Islets of Langerhans, appearing as lighter staining areas, were diffusely scattered throughout the pancreatic body. These islets house three crucial cell types: Alpha (α-cells) producing glucagon, primarily located around the islet periphery, with smaller size and a deep eosinophilic cytoplasm; Beta (β-cells) producing insulin, situated in the islet center with a pale basophilic cytoplasm; and Delta-cells (δ-cells) producing somatostatin, dispersed throughout the islets and characterized by small size, ovoid or ellipsoid nuclei, and a thin rim of eosinophilic cytoplasm. β-cells constitute approximately 60% of cells, α-cells 25%, and δ-cells only 5%. No inflammatory, degenerative, apoptotic, or necrotic changes were observed (Fig. 3A). Serial sections from the pancreatic tissue of the diabetic group (G2) displayed distinctive changes, including a reduction in islet cell densities and degenerative alterations in β-cells. These changes comprised cloudy swelling and hydropic degeneration, alongside necrotic and apoptotic changes in a subset of β-cells. Necrotic cells exhibited complete or partial loss of nuclear and/or cytoplasmic components, sometimes with ballooning changes. Apoptotic cells were characterized by small size, shrunken deep eosinophilic cytoplasm, and small nuclei. Exocrine pancreas changes were minimal, primarily marked by cystic dilatation and fibrosis of the affected ductal wall (Fig. 3B). In the diabetic rats treated with olive leaf extract or stem cells (G3 & G4), pancreatic alterations were mild, predictive, and promising. The Islets of Langerhans appeared comparatively larger with activated β-cells and a well-developed capillary network, resembling those in control negative rats (Figs.3C&D). A few β-cells exhibited apoptotic changes. The exocrine pancreas was nearly normal, with some cystically dilated pancreatic ducts containing intra-ductal secretory materials. Changes in pancreatic tissue observed in groups 4 and 5 were comparable to those in group 3, with very few degenerated and apoptotic β-cells noted in groups 4 and 5, respectively (Fig. 3D&E).
The fetal pancreas of the control group (G1) exhibits normal distribution and cellular densities. In the diabetic group (G2) and treatment groups (G3, 4, 5), there is a comparatively moderate decrease in cellular density compared to the control group, while cellular components gradually return to near-normal densities in the treatment groups. Across all groups, pancreatic structures appear primitive, with unclear cellular differentiation, particularly in the structural configuration of the Islets of Langerhans (Figs. 4).
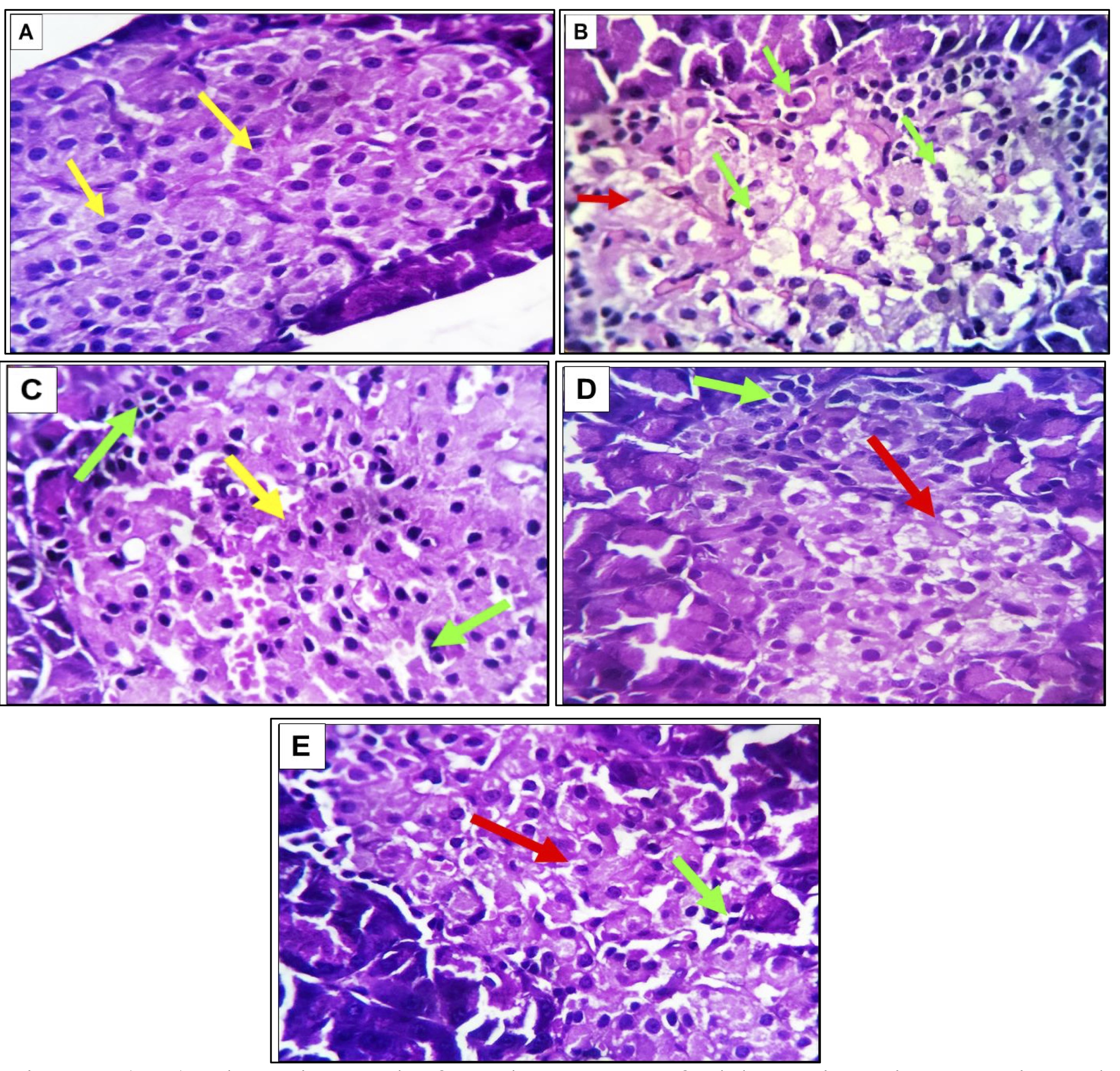
Fig. 3: (A-E). Photomicrographs from the pancreas of adult rats in various experimental groups. In G1 (A), the image illustrates a healthy acinar epithelium featuring well-defined secretory granules denoted by dark blue asterisks. The Islets of Langerhans, identified by light blue arrows, exhibit uniform distribution throughout the pancreas. These islets consist of α-cells with a deep eosinophilic cytoplasm and centrally located β-cells displaying a pale basophilic cytoplasm (yellow arrows). In G2 (B), a noticeable reduction in the density of islet cells (marked by red arrows) is observed, accompanied by degenerative necrotic changes. Moderate numbers of apoptotic alterations are evident in β-cells (highlighted green arrows) .G3(C) depicts enlarged Islets of Langerhans with activated β-cells and a well-developed capillary network (indicated by yellow arrows). While most β-cells exhibit vitality, a few show apoptotic changes (marked by green arrows). Groups 4 (D) and 5 €exhibit characteristics similar to those observed in Group 3, with very few degenerated and apoptotic β-cells in Group 5 (red and green arrows). The staining method employed is Hematoxylin and Eosin (H&E), with magnifications set at 100X and 400X.
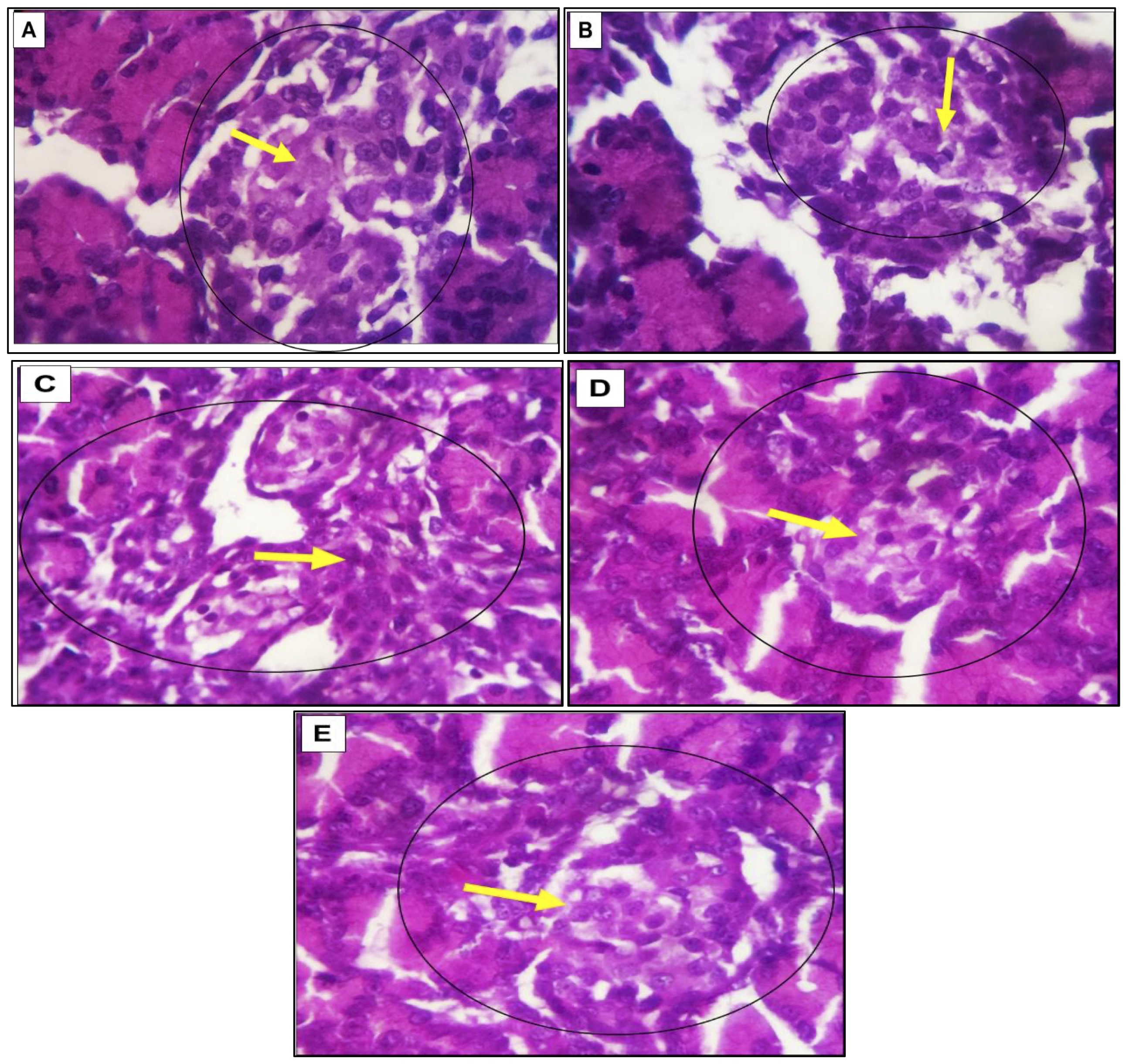
Fig. 4: (A-E). The photomicrograph illustrates images of the fetal rat pancreas from different experimental groups. The control group (G1) demonstrates a standard distribution and cellular densities (A). Conversely, the diabetic group (G2) exhibits a relatively moderate reduction in cellular density (B). In contrast, the three treatment groups (G3, G4, and G5) demonstrate a progressive recovery of cellular components, approaching normal densities. Across all groups, the pancreatic structures appear primitive, with unclear cellular differentiation, especially regarding the structural configuration of the islets of Langerhans (depicted by circles and yellow arrows) (C, D &E). The images were captured using H&E staining at magnifications of X 100 and 400.
Adult pancreas
In non-diabetic rats (G1), immunohistochemical examination revealed active positive cytoplasmic staining in nearly all β-cells within the islets of Langerhans. The stained cells exhibited a characteristic appearance, being large and rounded with centrally located nuclei and granular orange-red to dark brown cytoplasm. Conversely, α and δ-cells were negatively stained. In contrast, the pancreas of diabetic rats (G2) displayed a notable increase in positively stained cells with the insulin diabetic marker. The remaining cells exhibited negative staining, and other islet cells were also unstainable. treatment with olive leaf in diabetic rats (G3) resulted in a marked increase in positively stained β-cells for insulin markers. These positively stained cells exhibited a healthy condition with orange red to light brown cytoplasmic granules and large round nuclei. The remaining β-cells and other islet cells (α and δ-cells) were negatively stained. Stem cell treatment in diabetic rats (G4) showed nearly complete reactivity, with positively stained β-cells displaying orange-red to dark brown cytoplasmic reactivity. Combination therapy with olive leaf and stem cells in diabetic rats (G5) demonstrated a robust positive stainability for insulin markers in almost all cells (Fig. 5)
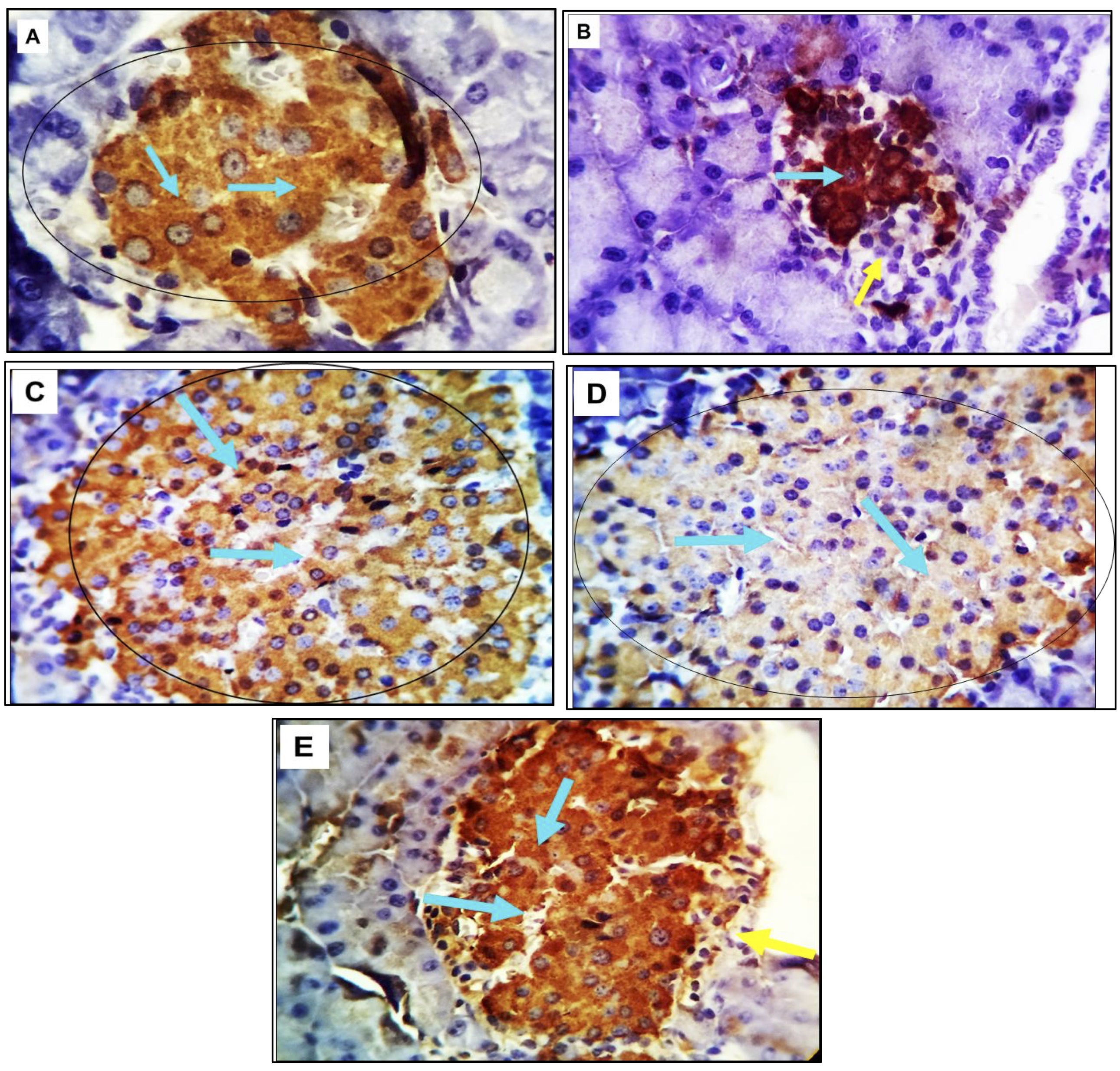
Fig. 5: (A-E). Photomicrograph depicting pancreatic tissue from adult rats across various experimental groups. The tissue was subjected to immunostaining with the diabetic marker insulin, revealing positive reactivity indicated by an orange red to brown cytoplasmic stainability in a variable number of cells (marked by light blue arrows). Conversely, unstained negative cells are denoted by yellow arrows. The magnification used for this image is 400X.
Fetal pancreas
Examination of the pancreas in non-diabetic fetal rats (Group 1) revealed active positive cytoplasmic staining in a moderate to marked number of Langerhans islet cells. The stained cells exhibited a large, rounded morphology with centrally located nuclei and granular orange red to dark brown cytoplasm. In contrast, the pancreas of diabetic rats (Group 2) exhibited a low to moderate number of positively reacting β-cells. Upon examination of pancreatic tissue from diabetic rats treated with olive leaf extract (Group 3), positive staining was observed in a moderate to marked number of β-cells for the insulin marker. These positive cells displayed orange red to dark brown cytoplasmic granules and large, round nuclei. Notably, sections from the pancreas of diabetic rats treated with stem cells (Group 4) showed a higher count of positively stained cells, particularly in near fully stained islets. These cells exhibited cytoplasmic reactivity ranging from orange red to dark brown. In the case of diabetic rats treated with both olive leaf extract and stem cells (Group 5), β-cells in the pancreatic tissue displayed a strong positive stainability for insulin markers in a moderate number of cells (Fig. 6).
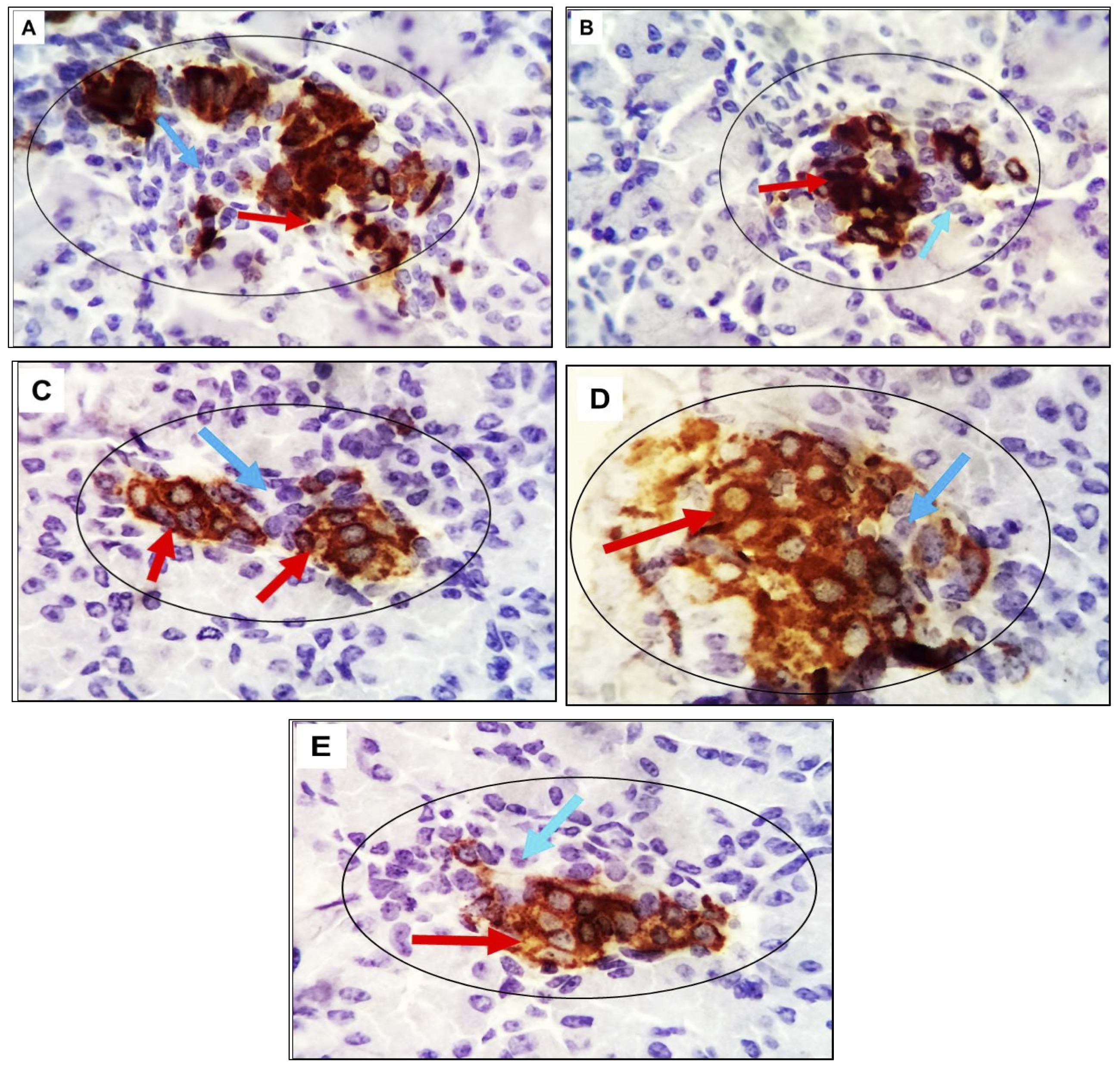
Fig. 6: (A-E): The photomicrograph of pancreatic tissue from rat fetuses across various experimental groups. The tissue is immune-stained with the diabetic marker insulin, revealing positive reactivity through an orange red to brown cytoplasmic stainability in a variable number of cells, as indicated by red arrows. Conversely, unstained negative cells are identified by light blue arrows. The magnification level is 400x.
Apoptotic marker P53
The pancreatic tissue sections from adult experimental rats were analyzed using immunohistochemistry with monoclonal antibodies targeting the apoptotic marker P53. Chromogen, in conjunction with Horseradish peroxidase (HRP), which can be conjugated to a labeled molecule, was employed as a developer. This labeled molecule, upon incubation with an appropriate substrate, generates a colored, fluorometric, or luminescent derivative, facilitating detection and quantification. HRP is commonly utilized in conjugate molecules that are genetically or chemically linkedto identify the presence of a molecular target. The results revealed that pancreatic tissue in the control group exhibited no apoptotic cells in the beta or alpha islet cells. In contrast, the pancreas of diabetic rats displayed a moderate number of immunopositively beta cells and a limited presence of alpha cells. Pancreatic beta and alpha cells in groups 3 and 4 tested negatives for the apoptotic marker P53. A minimal number of apoptotic beta cells were observed in group 5 (Fig. 7).
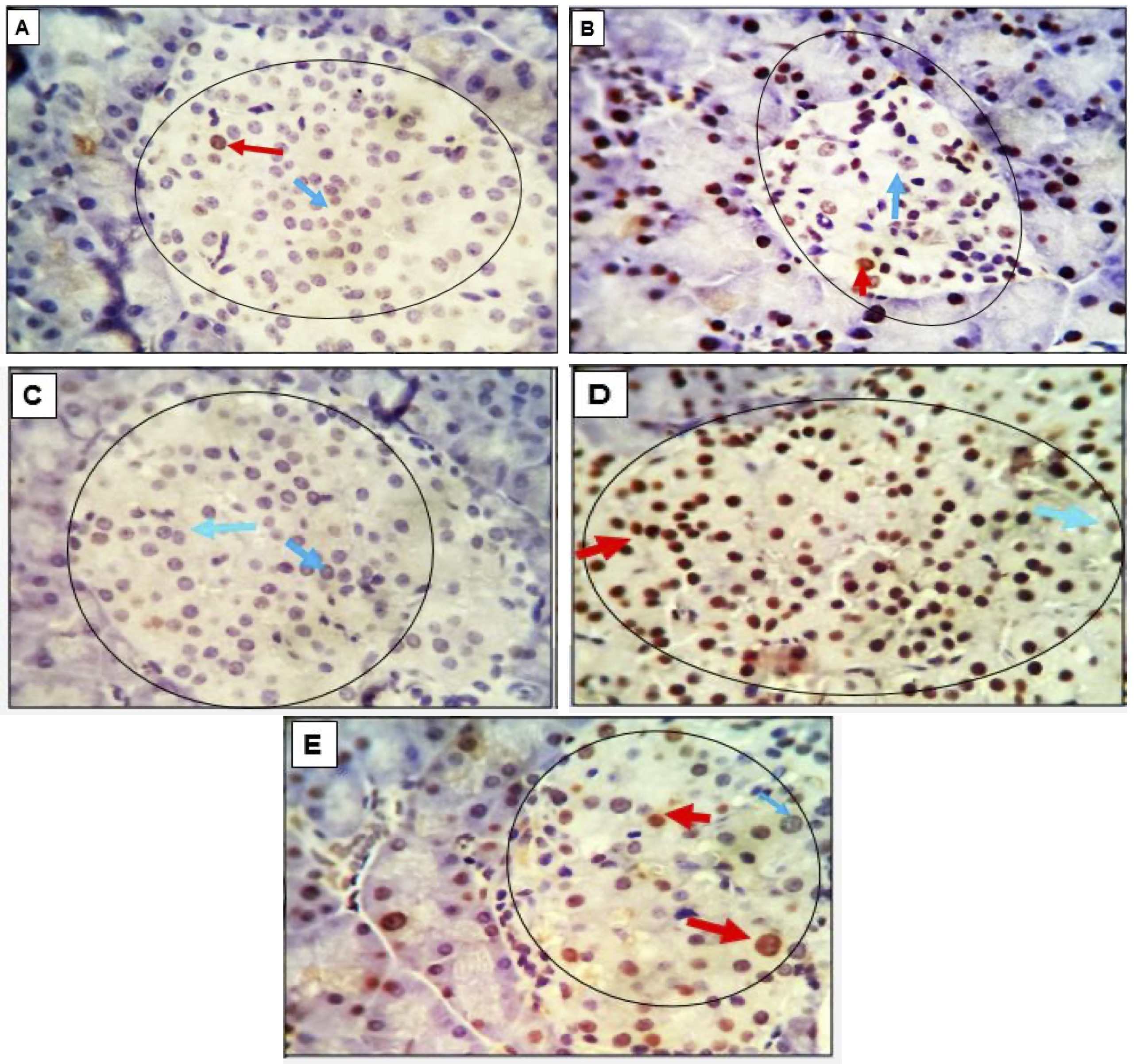
Fig. 7: (A-E): Photomicrograph of pancreatic tissue from adult rats belonging to various experimental groups. The specimens were immune stained with the apoptotic marker P53. The control group, characterized by normoglycemia, no apoptotic cells were observed in either the beta or alpha islet cells. In contrast, the pancreatic tissue of diabetic rats exhibited a moderate number of immune-positive beta cells and a limited presence of alpha cells. Unstained negative cells are identified by light blue arrows. The magnification level is 400x.
Proliferating cell nuclear antigen (PCNA)
The proliferating cell nuclear antigen (PCNA), a crucial component of the replication and repair machinery, plays a vital role in nucleic acid metabolism. The heightened expression of PCNA suggests that cells are experiencing damage and necessitate a compensatory proliferation response. Examination of pancreatic tissue in adult rats revealed minimal reactive cells with low intensity in the control group (G1), and the pancreatic tissue in G3 rats exhibited an entirely negative response. In G2 and G5, a few cells with moderate intensity were positively stained in the islets and exocrine pancreatic cells. Conversely, a moderate number of strongly positive cells were observed in G4 (Fig. 8).
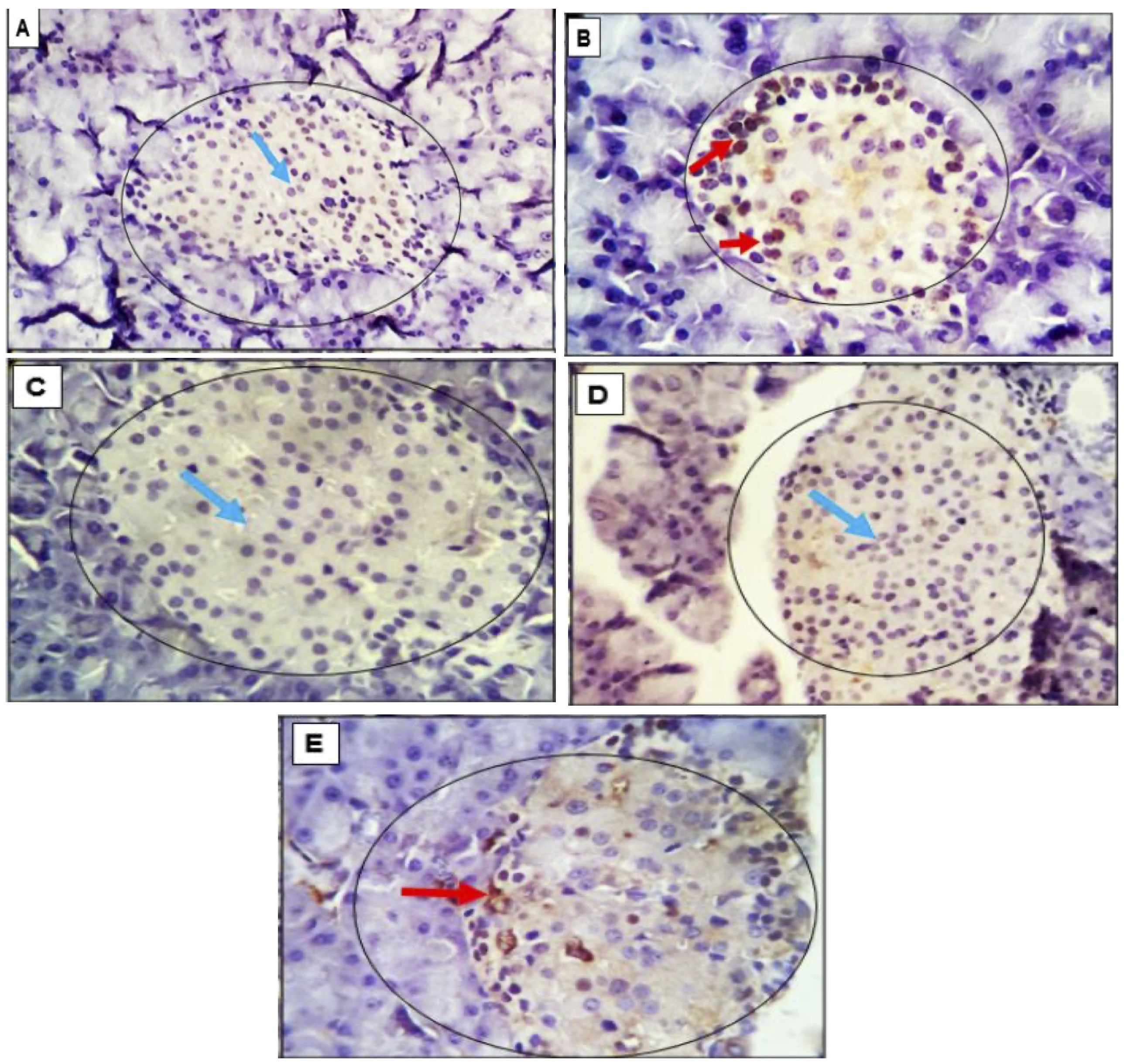
Fig. 8: (A-E): Photomicrograph assessing PCNA Expression in Pancreatic Tissue: Implications for Cell Damage and Proliferation Response in Adult Rats. The proliferating cell nuclear antigen (PCNA), a crucial component of the replication and repair machinery, plays a vital role in nucleic acid metabolism. The heightened expression of PCNA suggests that cells are experiencing damage and necessitate a compensatory proliferation response. Examination of pancreatic tissue in adult rats revealed minimal reactive cells with low intensity in the control group (A), and the pancreatic tissue in G3 rats (C) exhibited an entirely negative response. In G2 (B) and G5 (E), a few cells with moderate intensity were positively stained in the islets and exocrine pancreatic cells. Conversely, a moderate number of strongly positive cells were observed in G4 (D), unstained negative cells are identified by light blue arrows. The magnification level is 400x.
Discussion
As global living standards continue to improve, approximately 7% of pregnant women worldwide encounter gestational diabetes mellitus (GDM)[48]. GDM refers to the occurrence of impaired glucose tolerance associated with gestational changes, comprising more than 80% of diabetes mellitus cases during pregnancy [49]. The implementation of stem cell-based therapy introduces a novel treatment approach, exhibiting efficacy in animal models [50, 51].. Commonly employed agents include streptozotocin (STZ) and alloxan [52].. Consequently, STZ was chosen as the inducing agent in this investigation [53].
The hormonal and metabolic alterations in pregnant women diagnosed with gestational diabetes (GDM) substantially impact the intrauterine environment, resulting in irregular fetal development, pronounced metabolic consequences, and an increased risk of abnormal glucose tolerance and obesity during adolescence and adulthood. Considering the acknowledged benefits associated with the Mediterranean diet, our investigation focuses on assessing the efficacy of OLE, a crucial phenolic compound present in olives. Previous research has substantiated its safety for gravid mice [54].
In addition to manifestations of hyperglycemia, obesity, and insulin resistance, fetal development exhibited impairment in the specific genetic mouse model under investigation. Our study demonstrates that OLE effectively mitigated the elevated body weight observed in pregnant mice, concurrently leading to a reduction in blood glucose levels, alleviation of insulin resistance, and an increase in hepatic glycogen content. These findings align with previous research documenting the beneficial effects of OLE in lowering blood glucose levels in other models of gestational diabetes mellitus (GDM), including those induced by alloxan and obesity in diabetic rats [55].
The presented data yields valuable insights into the impact of various interventions on diverse parameters associated with diabetes. These findings carry significant implications for the effective management of diabetes and its related complications.
This research highlights an additional potential and beneficial effect of Olive Leaf Extract (OLE) in mitigating oxidative stress and reinforcing the body’s intrinsic defense mechanisms against antioxidants in diabetic rats already under oxidative stress. Moreover, this observation provides an additional basis for the hypoglycemic influence of OLE, attributable to its role as an antioxidant. Our findings contribute to a deeper understanding of how polyphenols participate in the regulation of glucose metabolism. OLE serves as an antioxidant in both preventive and remedial capacities. In terms of prevention, OLE has the potential to impede the formation of free radicals by binding to metal ions such as Cu and Fe, known catalysts for free radical generation [56], as well as by inhibiting various inflammatory enzymes like lipoxygenases [57]. In addressing the mitigation of pre-existing free radicals, the intervention of Olive Leaf Extract (OLE) may encompass the provision of hydroxyl groups, thereby directly neutralizing and quenching free radicals [58].
Maintaining glycemic control through the pursuit of defined HbA1c targets is recommended in accordance with current guidelines [59]. The statistical analysis conducted on the evaluated parameters revealed substantial disparities among the groups, particularly concerning glycemic control. The control group exhibited favorable HbA1c and fructosamine levels, indicative of a normoglycemic status. Conversely, the diabetic group demonstrated significantly elevated levels of both markers. The administration of olive extract and stem cell therapy resulted in noticeable reductions in both HbA1c and fructosamine levels. Notably, the combined treatment demonstrated a more pronounced decrease compared to each intervention alone. These outcomes strongly imply the potential efficacy of olive extract and stem cell therapy in enhancing glycemic control. These findings align with a previous study indicating the effectiveness of four plants, including olive leaves, used in traditional Arab medicine in managing blood glucose levels among diabetic patients [60, 61].Olive tree leaves have long been acknowledged as a traditional antidiabetic and antihypertensive herbal intervention in Europe, with historical use in treating infectious diseases [62, 63].Polyphenols derived from olive leaves have been identified as therapeutic agents capable of delaying the progression of inflammatory diseases mediated by advanced glycation end products, such as diabetes [64]. Moreover, OLE and tannins in olive leaves are reported to function as α-glucosidase inhibitors, reducing carbohydrate absorption in the gut. Studies on diabetic rats have demonstrated the inhibitory effect of olive leaf extract on postprandial blood glucose increase [65]. In human subjects treated with olive leaf extract, a significant decrease in blood glucose levels was observed after consuming cooked rice, compared to untreated controls [63, 65].
The results of the blood serum analysis for HbA1c indicate that the treatment groups positively impacted the reduction of hyperglycemia in diabetic rats by lowering the glycation of hemoglobin. A potential explanation for the unexpected outcome in the glibenclamide group is the severity of diabetes, notably reflected in fasting blood glucose levels exceeding 20 mmol/L. This level of severity may lead to significant beta cell damage, compromising the drug’s efficacy. This aligns with Sokolovska et al.’s study [66], which demonstrated that glibenclamide treatment (2 mg/kg over 6 weeks) in STZ-induced diabetic rats with blood glucose levels exceeding 13.89 mmol/L had no discernible impact on glycated hemoglobin levels due to substantial beta cell loss post-STZ administration [67].
The present study sheds light on the pivotal role of glucose levels as robust indicators for gauging the severity of diabetes. The observed substantial increase in glucose levels within the diabetic group underscores the gravity of their condition, a phenomenon well-documented in literature [68, 69]. However, our investigation reveals promising avenues for intervention, with both olive extract [70] and stem cell therapy [71] demonstrating an impressive ability to decrease glucose levels. This reduction, when further amplified through a combined treatment approach, suggests a potential synergistic interplay between these modalities, presenting a novel prospect for optimizing glucose balance [72]. Notably, this decline in glucose levels aligns with improvements in other measured parameters, highlighting a comprehensive enhancement in blood sugar management.
Insulin responsiveness, a defining feature of diabetes pathology measured through the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), revealed elevated values within the diabetic cohort, indicative of insulin resistance [73]. In agreement with existing literature, our findings accentuate the efficiency of both olive extract [68, 70] and stem cell therapy [71]in mitigating insulin resistance, thereby emphasizing their potential to enhance insulin sensitivity. The comparable HOMA-IR values observed in the combined treatment group, mirroring those of the control group, provide compelling evidence for the potency of this approach in reversing insulin resistance [74]. This decline in insulin resistance aligns cohesively with the positive changes witnessed in other glycemic measures, further substantiating the therapeutic promise of these interventions [43, 75]. While our study advances our understanding of these treatments, it is imperative to acknowledge potential criticisms and further explore the intricate molecular mechanisms underlying these observed effects, opening avenues for future research [76].
Olive extract, MSCs, and the combined treatment led to improved HOMA-IR values, suggesting enhanced insulin sensitivity in diabetic rats. This finding implies that the groups of MSCs and the olive leaf might regenerate or repair damaged pancreatic islets in diabetic rats. According to Sun et al. [77], a concentration of 10 mg/kg of hUC-MSCs-sEVs could alleviate islet damage in T2DM rats by inhibiting STZ-induced apoptosis. Consequently, a histological assessment of pancreatic islets is warranted.
In the context of gestational diabetes mellitus (GDM) development, inflammation and oxidative stress emerge as pivotal factors. Our investigation reveals that the application of OLE treatment possesses the capability to ameliorate the heightened inflammatory response and oxidative stress. Variations in the inflammatory response and oxidative stress during pregnancy may be linked to alterations in hormone levels and energy metabolism in expectant mothers [78]. Elevated hormone production has the potential to instigate the breakdown of fats, potentially disrupting the release of inflammatory agents, lipid metabolism, glucose metabolism, and insulin resistance [79]. Aberrant protein and lipid oxidation may also contribute to excessive oxidative stress, further disturbing the body’s homeostasis [80].
The hormone profile analysis conducted in this study unveiled significant disparities among the diverse groups. Notably, the diabetic group exhibited distinct hormonal changes compared to the control group.
Thyroid-Stimulating Hormone (TSH) Levels: The diabetic group displayed higher TSH levels than the control group, suggesting potential thyroid dysfunction in diabetes. Intriguingly, the introduction of stem cell therapy appeared to effectively lower TSH levels, approaching those of the control group. This implies a promising role of stem cell therapy in regulating thyroid function in diabetics.
Building upon Feng et al.’s [81] findings, observed similar trends in their study, reinforcing the potential link between diabetes and thyroid dysfunction. Furthermore, [82] supported these conclusions in their meta-analysis, emphasizing the need for innovative interventions. The consistent alignment of these studies underscores the promising prospect of stem cell therapy as a viable approach to modulate thyroid-stimulating hormone levels in diabetic individuals.
Building upon Abd El-Hakim et al.’s findings [83], proposed that the observed improvement in FSH levels among the diabetic group supplemented with olive extract may be attributed to the antioxidant properties of olive compounds. Additionally, [84] support these results, emphasizing the potential of stem cell therapy in regulating hormonal imbalances, reinforcing the idea that multi-modal interventions hold promise for restoring hormonal equilibrium in diabetic individuals.
The observed increase in LH levels following the supplementation of olive extract and stem cells aligns with findings by [85], who demonstrated the positive impact of olive polyphenols on endocrine function. Furthermore, the synergistic effect noted in the combination intervention emphasizing the potential of combined therapies in optimizing hormonal balance within the hypothalamic-pituitary-gonadal axis.
Prolactin (PRL) Levels: The findings align with previous research byManshaei et al. [2], who highlighted the potential of olive extract in ameliorating diabetic conditions. Additionally,Tesone et al. [86] demonstrated the efficacy of stem cell interventions in hormonal balance. These consistent trends in PRL-level recovery across various interventions underscore the multi-faceted approach to diabetes management, as emphasized byAlejandro et al. [87].
Building on the findings,Alipio et al. [88] proposed that stem cell therapy could serve as a promising avenue for addressing progesterone deficiencies in diabetic individuals. Furthermore, Szunerits et al. [89] highlighted the synergistic effect observed when combining stem cell therapy with olive extract, reinforcing the significance of multi-faceted interventions in managing hormonal imbalances linked to diabetes. These collective insights not only corroborate the study’s results but also suggest a broader application of such strategies in hormonal regulation for diabetic patients.
Building on the research by [90], who highlighted the anti-inflammatory properties of olive extract, and the work of Ambrosi et al. [91], showcasing the regenerative capabilities of stem cells, our findings align with their insights, emphasizing the potential of these interventions in diabetes management. Moreover, the collaborative study byMills et al. [92], which explored the interplay of hormones, provides a theoretical framework supporting our observed modulatory effects on thyroid, gonadal, and prolactin hormones.
The present study delves into the histological alterations within the pancreatic islets of adult rats under different experimental conditions. Our detailed examination of α-cells, β-cells, and δ-cells has provided insights into the nuanced changes associated with glucose homeostasis. These findings align with the work of [93, 94], who similarly highlighted the pivotal role of these cell types in pancreatic function.
Our observations of the diabetic group (G2) echo the findings of [95], revealing a decrease in islet cell densities and degenerative changes in β-cells. These alterations underscore the impact of diabetes on pancreatic tissue, particularly on the essential β-cell function. Dai’s work supports our findings, linking compromised β-cell function with cellular changes observed in diabetic conditions [96].
Contrastingly, the treatment groups (G3, G4, G5) demonstrated noteworthy improvements. The positive outcomes, including enlarged islets, activated β-cells, and a robust capillary network, align with the restorative potential of the applied interventions [97]. proposed similar treatments, supporting our assertion that these interventions may contribute to the amelioration of diabetic-induced histological changes.
Fetal Histopathology: The intriguing observation of a gradual recovery in cellular densities across treatment groups hints at the potential therapeutic efficacy. The primitive appearance of pancreatic structures, however, prompts further investigation [23, 98] have previously discussed the challenges associated with deciphering fetal pancreatic differentiation, emphasizing the need for caution in interpreting such observations.
Our immuno-histochemical analysis, focusing on insulin markers, aligns with the work of [99], who emphasized the importance of insulin-positive staining in healthy β-cells. The robust staining in treated groups (G4 and G5) suggests a potential therapeutic effect on β-cell function. Moreover, our findings on P53-positive cells corroborate with [100], supporting the hypothesis that our treatment strategies may protect against apoptosis.
Examining PCNA expression, our results indicate varying degrees of cellular proliferation response in different experimental conditions. The compensatory proliferation observed in group 4 resonates with [101] Wang’s discussions on adaptive responses to cellular damage. Conversely, the lack of PCNA staining in group 3 raises questions and warrants further exploration into the dynamics of proliferation response under this specific treatment [102, 103]. have highlighted the intricate balance required for pancreatic cellular proliferation, emphasizing the need for nuanced interpretations.
The standard of care for GDM primarily involves lifestyle modifications, including diet and exercise, as first-line interventions. If these measures do not achieve adequate glycemic control, pharmacologic treatments such as insulin therapy or oral medications (e.g., metformin or glyburide) are typically prescribed to maintain blood glucose within target ranges. However, these treatments have limitations, including risks of maternal hypoglycemia and potential adverse fetal effects. The findings of this study suggest that combining mesenchymal stem cell therapy and olive leaf extract could offer an alternative or adjunctive approach, potentially enhancing glycemic control and improving pancreatic health while reducing dependence on conventional drug therapies. Further studies are needed to explore how these interventions could be integrated into clinical management.
Conclusion
In conclusion, this comprehensive study underscores the potential of olive leaf extract and stem cell therapy, both individually and in combination, as promising interventions in managing gestational diabetes mellitus and diabetes-related complications. The research demonstrates their efficacy in improving glycemic control, insulin sensitivity, and hormonal balance, while also exhibiting positive effects on pancreatic islet dynamics and fetal histopathology. The observed synergistic interactions between these modalities open new avenues for optimizing diabetes management. Although the study provides valuable insights, further research is warranted to delve into the intricate molecular mechanisms underlying these effects and to explore the broader application of these strategies in diverse diabetic populations.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research in cooperation with the Olive Research Center at Jouf University for funding this work through research grant no. DSR2022-RG-0165.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Funding
This work was funded by the Deanship of Scientific Research in cooperation with the Olive Research Center at Jouf University, under Grant Number (DSR2022-RG-0165).
Disclosure Statement
The authors have nothing to disclose.
AI disclosure
No AI tools have been used to create this work.
References
| 1 | Olvera-Roldán EO, Cristóbal-Luna JM, García-Martínez Y, Mojica-Villegas MA, Pérez-Pastén-Borja R, Gutiérrez-Salmeán G, Pérez-Gutiérrez S, García-Rodríguez RV, Madrigal-Santillán E, Morales-González JA, Chamorro-Cevallos G: Effects of Spirulina maxima on a Model of Sexual Dysfunction in Streptozotocin-Induced Diabetic Male Rats. Plants. DOI: 10.3390/plants12040722.
https://doi.org/10.3390/plants12040722 |
| 2 | Manshaei N, Shakibaei F, Fazilati M, Salavati H, Negahdary M, Palizban A: An investigation of the association between the level of prolactin in serum and type II diabetes. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2019;13:3035-3041.
https://doi.org/10.1016/j.dsx.2018.07.007 |
| 3 | Ghaheri M, Miraghaee S, Babaei A, Mohammadi B, Kahrizi D, Saivosh Haghighi ZM, Bahrami G: Effect of Stevia rebaudiana Bertoni extract on sexual dysfunction in Streptozotocin-induced diabetic male rats. Cellular and molecular biology (Noisy-le-Grand, France) 2018;64:6-10.
https://doi.org/10.14715/cmb/2018.64.2.2 |
| 4 | Algaba-Chueca F, Maymó-Masip E, Ejarque M, Ballesteros M, Llauradó G, López C, Guarque A, Serena C, Martínez-Guasch L, Gutiérrez C, Bosch R, Vendrell J, Megía A, Fernández-Veledo S: Gestational diabetes impacts fetal precursor cell responses with potential consequences for offspring. Stem Cells Translational Medicine 2020;9:351-363.
https://doi.org/10.1002/sctm.19-0242 |
| 5 | Wang L, O'Kane AM, Zhang Y, Ren J: Maternal obesity and offspring health: Adapting metabolic changes through autophagy and mitophagy. Obesity Reviews 2023:e13567.
https://doi.org/10.1111/obr.13567 |
| 6 | Sferruzzi-Perri AN, Lopez-Tello J, Salazar-Petres E: Placental adaptations supporting fetal growth during normal and adverse gestational environments. Experimental Physiology 2023;108:371-397.
https://doi.org/10.1113/EP090442 |
| 7 | Carobbio S, Guénantin A-C, Samuelson I, Bahri M, Vidal-Puig A: Brown and beige fat: from molecules to physiology and pathophysiology. Biochimica et Biophysica Acta -Molecular and Cell Biology of Lipids 2019;1864:37-50.
https://doi.org/10.1016/j.bbalip.2018.05.013 |
| 8 | Friedman JE: Developmental programming of obesity and diabetes in mouse, monkey, and man in 2018: where are we headed? Diabetes 2018;67:2137-2151.
https://doi.org/10.2337/dbi17-0011 |
| 9 | Joshi NP, Madiwale SD, Sundrani DP, Joshi SR: Fatty acids, inflammation and angiogenesis in women with gestational diabetes mellitus. Biochimie 2023;212:31-40.
https://doi.org/10.1016/j.biochi.2023.04.005 |
| 10 | Mahmoud M, Abu-Shahba N, Azmy O, El-Badri N: Impact of diabetes mellitus on human mesenchymal stromal cell biology and functionality: implications for autologous transplantation. Stem cell reviews and reports 2019;15:194-217.
https://doi.org/10.1007/s12015-018-9869-y |
| 11 | Rakieten N: Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemotherapy Researches 1963;29:91-98.
|
| 12 | Junod A, Lambert AE, Stauffacher W, Renold AE: Diabetogenic action of streptozotocin: relationship of dose to metabolic response. The Journal of clinical investigation 1969;48:2129-2139.
https://doi.org/10.1172/JCI106180 |
| 13 | Junod A, Lambert AE, Orci L, Pictet R, Gonet AE, Renold AE: Studies of the Diabetogenic Action of Streptozotocin. Proceedings of the Society for Experimental Biology and Medicine 1967;126:201-205.
https://doi.org/10.3181/00379727-126-32401 |
| 14 | Furman BL: Streptozotocin-Induced Diabetic Models in Mice and Rats. Current Protocols 2021;1:e78.
https://doi.org/10.1002/cpz1.78 |
| 15 | Le TT, Trang NT, Pham VTT, Quang DN, Phuong Hoa LT: Bioactivities of β-mangostin and its new glycoside derivatives synthesized by enzymatic reactions. Royal Society Open Science 2023;10:230676.
https://doi.org/10.1098/rsos.230676 |
| 16 | Huang J, Yeung AM, Nguyen KT, Xu NY, Preiser J-C, Rushakoff RJ, Seley JJ, Umpierrez GE, Wallia A, Drincic A: Hospital Diabetes Meeting 2022. Journal of diabetes science and technology 2022;16:1309-1337.
https://doi.org/10.1177/19322968221110878 |
| 17 | Aierken A, Li B, Liu P, Cheng X, Kou Z, Tan N, Zhang M, Yu S, Shen Q, Du X, Enkhbaatar BB, Zhang J, Zhang R, Wu X, Wang R, He X, Li N, Peng S, Jia W, Wang C, Hua J: Melatonin treatment improves human umbilical cord mesenchymal stem cell therapy in a mouse model of type II diabetes mellitus via the PI3K/AKT signaling pathway. Stem Cell Research & Therapy 2022;13:164.
https://doi.org/10.1186/s13287-022-02832-0 |
| 18 | Luo L, Zhou Y, Zhang C, Huang J, Du J, Liao J, Bergholt NL, Bünger C, Xu F, Lin L: Feeder-free generation and transcriptome characterization of functional mesenchymal stromal cells from human pluripotent stem cells. Stem Cell Research & Therapy 2020;48:101990.
https://doi.org/10.1016/j.scr.2020.101990 |
| 19 | Li N, Hua J: Interactions between mesenchymal stem cells and the immune system. Cellular and Molecular Life Sciences 2017;74:2345-2360.
https://doi.org/10.1007/s00018-017-2473-5 |
| 20 | Nazhvani FD, Haghani I, Nazhvani SD, Namazi F, Ghaderi A: Regenerative effect of mesenteric fat stem cells on ccl4-induced liver cirrhosis, an experimental study. Annals of Medicine and Surgery 2020;60:135-139.
https://doi.org/10.1016/j.amsu.2020.10.045 |
| 21 | Mohsen ROM, Halawa AM, Hassan R: Role of bone marrow-derived stem cells versus insulin on filiform and fungiform papillae of diabetic albino rats (light, fluorescent and scanning electron microscopic study). Acta Histochemica 2019;121:812-822.
https://doi.org/10.1016/j.acthis.2019.07.007 |
| 22 | Husakova J, Echalar B, Kossl J, Palacka K, Fejfarova V, Dubsky M: The Effects of Immunosuppressive Drugs on the Characteristics and Functional Properties of Bone Marrow-Derived Stem Cells Isolated from Patients with Diabetes Mellitus and Peripheral Arterial Disease. Biomedicines 2023;11:1872.
https://doi.org/10.3390/biomedicines11071872 |
| 23 | El-Sayed ME, Atwa A, Sofy AR, Helmy YA, Amer K, Seadawy MG, Bakry S: Mesenchymal stem cell transplantation in burn wound healing: uncovering the mechanisms of local regeneration and tissue repair. Histochemistry and Cell Biology 2023
https://doi.org/10.1007/s00418-023-02244-y |
| 24 | Fang J, Yan Y, Teng X, Wen X, Li N, Peng S, Liu W, Donadeu FX, Zhao S, Hua J: Melatonin prevents senescence of canine adipose-derived mesenchymal stem cells through activating NRF2 and inhibiting ER stress. Aging 2018;10:2954.
https://doi.org/10.18632/aging.101602 |
| 25 | Atwa A, Latif AKMA, Moustafa MA, Ashry M, Askar H, Shehata AZI, Mehany ABM, Hallool SI, Bakry S: Integrating Nanosensors into Stem Cells Technologies and Regenerative Medicine; Handbook of Nanosensors: Materials and Technological Applications, 2024, pp 1113-1147.
https://doi.org/10.1007/978-3-031-47180-3_38 |
| 26 | Schwartz DD, Stewart SD, Aikens JE, Bussell JK, Osborn CY, Safford MM: Seeing the Person, Not the Illness: Promoting Diabetes Medication Adherence Through Patient-Centered Collaboration. Clinical Diabetes 2017;35:35-42.
https://doi.org/10.2337/cd16-0007 |
| 27 | Aryaeian N, Sedehi SK, Arablou T: Polyphenols and their effects on diabetes management: A review. Medical journal of the Islamic Republic of Iran 2017;31:134.
https://doi.org/10.14196/mjiri.31.134 |
| 28 | Zinjarde SS, Bhargava SY, Kumar AR: Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC complementary and alternative medicine 2011;11:1-10.
https://doi.org/10.1186/1472-6882-11-5 |
| 29 | Boskou D: Olive and olive oil bioactive constituents. Elsevier, 2015.
https://doi.org/10.1016/B978-1-63067-041-2.50007-0 |
| 30 | Romani A, Ieri F, Urciuoli S, Noce A, Marrone G, Nediani C, Bernini R: Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019;11:1776.
https://doi.org/10.3390/nu11081776 |
| 31 | Siddiqui SA, Khan S, Wani SA: Controlling diabetes with the aid of medicinal herbs: a critical compilation of a decade of research. Critical Reviews in Food Science and Nutrition 2022:1-15.
https://doi.org/10.1080/10408398.2022.2103088 |
| 32 | Abdel-Reheim ES, Abd-Elmoneim AA, Hosni AA: Fatty-sucrosed diet/minimal dose of streptozotocin-treated rat: a novel model of gestational diabetes mellitus, metabolic and inflammatory insight. Journal of Diabetes and Metabolism 2014;5
|
| 33 | Suzuki YJ, Jain V, Park A-M, Day RM: Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radical Biology and Medicine 2006;40:1683-1692.
https://doi.org/10.1016/j.freeradbiomed.2006.01.008 |
| 34 | Wang Y-h, Zheng R, Chen L: Isolation and culture of rat bone marrow mesenchymal stem cells using density gradient centrifugation and adherence separation screening. Chinese Journal of Tissue Engineering Research 2014;18:4463.
|
| 35 | Harting M, Jimenez F, Pati S, Baumgartner J, Cox Jr C: Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy 2008;10:243-253.
https://doi.org/10.1080/14653240801950000 |
| 36 | Furman BL: Streptozotocin‐induced diabetic models in mice and rats. Current protocols in pharmacology 2015;70:5.47. 41-45.47. 20.
https://doi.org/10.1002/0471141755.ph0547s70 |
| 37 | Trinder P: Determination of Glucose in Blood Using Glucose Oxidase with an Alternative Oxygen Acceptor. Annals of Clinical Biochemistry 1969;6:24-27.
https://doi.org/10.1177/000456326900600108 |
| 38 | Armbruster DA: Fructosamine: structure, analysis, and clinical usefulness. Clinical Chemistry 1987;33:2153-2163.
https://doi.org/10.1093/clinchem/33.12.2153 |
| 39 | Matthews DR, Bown E, Beck TW, Plotkin E, Lock L, Gosden E, Wickham M: An Amperometric Needle-type Glucose Sensor Tested in Rats and Man. Diabetic Medicine 1988;5:248-252.
https://doi.org/10.1111/j.1464-5491.1988.tb00978.x |
| 40 | Genovesi S, Montelisciani L, Giussani M, Lieti G, Patti I, Orlando A, Antolini L, Parati G: Role of Insulin Resistance as a Mediator of the Relationship between Body Weight, Waist Circumference, and Systolic Blood Pressure in a Pediatric Population. Metabolites. DOI: 10.3390/metabo13030327.
https://doi.org/10.3390/metabo13030327 |
| 41 | Leirs K, Dal Dosso F, Perez-Ruiz E, Decrop D, Cops R, Huff J, Hayden M, Collier N, Yu KXZ, Brown S, Lammertyn J: Bridging the Gap between Digital Assays and Point-of-Care Testing: Automated, Low Cost, and Ultrasensitive Detection of Thyroid Stimulating Hormone. Analytical Chemistry 2022;94:8919-8927.
https://doi.org/10.1021/acs.analchem.2c00480 |
| 42 | Bablok L, Janczewski Z, Kwiatkowska Z, Fracki S: The Relationship between Plasma FSH, Testosterone Levels and Testicular Histology in Males with Azoospermia. Andrologia 1978;10:502-505.
https://doi.org/10.1111/j.1439-0272.1978.tb03084.x |
| 43 | Pedersen SB, Kristensen K, Richelsen B: Anti-glucocorticoid effects of progesterone in vivo on rat adipose tissue metabolism. Steroids 2003;68:543-550.
https://doi.org/10.1016/S0039-128X(03)00084-9 |
| 44 | Suvarna KS, Layton C, Bancroft JD: Bancroft's theory and practice of histological techniques. Elsevier health sciences, 2018.
|
| 45 | Janardhan KS, Jensen H, Clayton NP, Herbert RA: Immunohistochemistry in Investigative and Toxicologic Pathology. Toxicologic Pathology 2018;46:488-510.
https://doi.org/10.1177/0192623318776907 |
| 46 | Mok JX, Ooi JH, Ng KY, Koh RY, Chye SM: A new prospective on the role of melatonin in diabetes and its complications. Hormone Molecular Biology and Clinical Investigation 2019;40:20190036.
https://doi.org/10.1515/hmbci-2019-0036 |
| 47 | Armonk: v. 23.0, IBM Corp., Armonk, NY, USA; 2015, 2015,
|
| 48 | Ketumarn N, Boriboonhirunsarn D: Characteristics of abnormal oral glucose tolerance test in GDM diagnosis and clinical correlation. The Journal of Maternal-Fetal & Neonatal Medicine 2018;31:2109-2114.
https://doi.org/10.1080/14767058.2017.1336224 |
| 49 | Carolan-Olah MC: Educational and intervention programmes for gestational diabetes mellitus (GDM) management: An integrative review. Collegian 2016;23:103-114.
https://doi.org/10.1016/j.colegn.2015.01.001 |
| 50 | Lavrenko AV, Rasin MS, Kutsenko LA, Mikitiuk MV, Solokhina IL, Mamontova TV, Kaĭdashev IP: [Efficacy of short-term metformin application in complex treatment of patients with coronary artery disease and concomitant metabolic syndrome]. Lik Sprava 2010:65-71.
|
| 51 | Sameni HR, Ramhormozi P, Bandegi AR, Taherian AA, Mirmohammadkhani M, Safari M: Effects of ethanol extract of propolis on histopathological changes and anti-oxidant defense of kidney in a rat model for type 1 diabetes mellitus. Journal of Diabetes Investigation 2016;7:506-513.
https://doi.org/10.1111/jdi.12459 |
| 52 | Gundala NKV, Naidu VGM, Das UN: Arachidonic acid and lipoxin A4 attenuate alloxan-induced cytotoxicity to RIN5F cells in vitro and type 1 diabetes mellitus in vivo. BioFactors 2017;43:251-271.
https://doi.org/10.1002/biof.1336 |
| 53 | Wang Y, Feng Q, Niu X, Xu K, Wang Y, Wang J, Li Q, Mao Y, Gao S: The Preventive Effect of Zuogui Wan on Offspring Rats' Impaired Glucose Tolerance Whose Mothers Had Gestational Diabetes Mellitus. Evidence-Based Complementary and Alternative Medicine 2016;2016:9417362.
https://doi.org/10.1155/2016/9417362 |
| 54 | Mohammed HA, Okail HA, Ibrahim MA, Emam NM: Influences of olive leaf extract in the kidney of diabetic pregnant mice and their offspring. Journal of Basic and Applied Zoology 2018;79:1-13.
https://doi.org/10.1186/s41936-018-0024-8 |
| 55 | Al-Azzawie HF, Alhamdani MS: Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sciences 2006;78:1371-1377.
https://doi.org/10.1016/j.lfs.2005.07.029 |
| 56 | Andrikopoulos NK, Kaliora AC, Assimopoulou AN, Papageorgiou VP: Inhibitory Activity of Minor Polyphenolic and Nonpolyphenolic Constituents of Olive Oil Against In vitro Low-Density Lipoprotein Oxidation. Journal of Medicinal Food 2002;5:1-7.
https://doi.org/10.1089/109662002753723160 |
| 57 | Allegretta C, Difonzo G, Caponio F, Tamma G, Laselva O: Olive Leaf Extract (OLE) as a Novel Antioxidant That Ameliorates the Inflammatory Response in Cystic Fibrosis. Cells. DOI: 10.3390/cells12131764.
https://doi.org/10.3390/cells12131764 |
| 58 | Visioli F, Poli A, Gall C: Antioxidant and other biological activities of phenols from olives and olive oil. Medicinal Research Reviews 2002;22:65-75.
https://doi.org/10.1002/med.1028 |
| 59 | Woo V, Shestakova MV, Ørskov C, Ceriello A: Targets and tactics: the relative importance of HbA1c, fasting and postprandial plasma glucose levels to glycaemic control in type 2 diabetes. International Journal of Clinical Practice 2008;62:1935-1942.
https://doi.org/10.1111/j.1742-1241.2008.01941.x |
| 60 | Huseini HF, Larijani B, Heshmat R, Fakhrzadeh H, Radjabipour B, Toliat T, Raza M: The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytotherapy Research 2006;20:1036-1039.
https://doi.org/10.1002/ptr.1988 |
| 61 | Said O, Fulder S, Khalil K, Azaizeh H, Kassis E, Saad B: Maintaining a Physiological Blood Glucose Level with 'Glucolevel', a Combination of Four Anti-Diabetes Plants Used in the Traditional Arab Herbal Medicine. Evidence-Based Complementary and Alternative Medicine 2008;5:720130.
https://doi.org/10.1093/ecam/nem047 |
| 62 | Gonzalez M, Zarzuelo A, Gamez MJ, Utrilla MP, Jimenez J, Osuna I: Hypoglycemic Activity of Olive Leaf. Planta Med 1992;58:513-515.
https://doi.org/10.1055/s-2006-961538 |
| 63 | Wainstein J, Ganz T, Boaz M, Bar Dayan Y, Dolev E, Kerem Z, Madar Z: Olive Leaf Extract as a Hypoglycemic Agent in Both Human Diabetic Subjects and in Rats. Journal of Medicinal Food 2012;15:605-610.
https://doi.org/10.1089/jmf.2011.0243 |
| 64 | Chandler D, Woldu A, Rahmadi A, Shanmugam K, Steiner N, Wright E, Benavente-García O, Schulz O, Castillo J, Münch G: Effects of plant-derived polyphenols on TNF-α and nitric oxide production induced by advanced glycation endproducts. Molecular Nutrition & Food Research 2010;54:S141-S150.
https://doi.org/10.1002/mnfr.200900504 |
| 65 | Komaki E, Yamaguchi S, Maru I, Kinoshita M, Kakehi K, Ohta Y, Tsukada Y: Identification of Anti-α-Amylase Components from Olive Leaf Extracts. Food Science and Technology Research 2003;9:35-39.
https://doi.org/10.3136/fstr.9.35 |
| 66 | Sokolovska J, Isajevs S, Sugoka O, Sharipova J, Paramonova N, Isajeva D, Rostoka E, Sjakste T, Kalvinsh I, Sjakste N: Comparison of the Effects of Glibenclamide on Metabolic Parameters, GLUT1 Expression, and Liver Injury in Rats With Severe and Mild Streptozotocin-Induced Diabetes Mellitus. Medicina. DOI: 10.3390/medicina48100078.
https://doi.org/10.3390/medicina48100078 |
| 67 | Ooi DJ, Adamu HA, Imam MU, Ithnin H, Ismail M: Polyphenol-rich ethyl acetate fraction isolated from Molineria latifolia ameliorates insulin resistance in experimental diabetic rats via IRS1/AKT activation. Biomedicine & Pharmacotherapy 2018;98:125-133.
https://doi.org/10.1016/j.biopha.2017.12.002 |
| 68 | Sharma V, Mehdi MM: Oxidative stress, inflammation and hormesis: The role of dietary and lifestyle modifications on aging. Neurochemistry International 2023;164:105490.
https://doi.org/10.1016/j.neuint.2023.105490 |
| 69 | Grammes J, Stock W, Mann CG, Flynn EM, Kubiak T: Focus group study to identify the central facets of fear of hypoglycaemia in people with Type 2 diabetes mellitus. Diabetic Medicine 2017;34:1765-1772.
https://doi.org/10.1111/dme.13506 |
| 70 | Casado-Díaz A, Dorado G, Quesada-Gómez JM: Influence of olive oil and its components on mesenchymal stem cell biology. World journal of stem cells 2019;11:1045-1064.
https://doi.org/10.4252/wjsc.v11.i12.1045 |
| 71 | Tezcan G, Tunca B, Bekar A, Budak F, Sahin S, Cecener G, Egeli U, Taskapılıoglu MO, Kocaeli H, Tolunay S, Malyer H, Demir C, Tumen G: Olea europaea leaf extract improves the treatment response of GBM stem cells by modulating miRNA expression. American journal of cancer research 2014;4:572-590.
|
| 72 | Bahl V, Lee May C, Perez A, Glaser B, Kaestner KH: Genetic activation of α-cell glucokinase in mice causes enhanced glucose-suppression of glucagon secretion during normal and diabetic states. Molecular Metabolism 2021;49:101193.
https://doi.org/10.1016/j.molmet.2021.101193 |
| 73 | Owei I, Umekwe N, Provo C, Wan J, Dagogo-Jack S: Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: role in prediction of incident pre-diabetes in a longitudinal biracial cohort. 2017;5:e000415.
https://doi.org/10.1136/bmjdrc-2017-000415 |
| 74 | Atef MM, Abd-Ellatif RN, Emam MN, Abo El gheit RE, Amer AI, Hafez YM: Therapeutic potential of sodium selenite in letrozole induced polycystic ovary syndrome rat model: Targeting mitochondrial approach (selenium in PCOS). Archives of Biochemistry and Biophysics 2019;671:245-254.
https://doi.org/10.1016/j.abb.2019.06.009 |
| 75 | Atwa A, Sofy MR, Fakhrelden SM, Darwish O, Mehany ABM, Sofy AR, Bakry S: Biodegradable Materials from Natural Origin for Tissue Engineering and Stem Cells Technologies; Handbook of Biodegradable Materials, 2023, pp 1133-1172.
https://doi.org/10.1007/978-3-031-09710-2_63 |
| 76 | Ashry M, Askar H, Obiedallah MM, Elankily AH, Galal El-Sahra D, Zayed G, Mustafa MA, El-Shamy SAEM, Negm SA, El-Beltagy MA, Abdel-Wahhab KG, Ene A: Hormonal and inflammatory modulatory effects of hesperidin in hyperthyroidism-modeled rats. Frontiers in Immunology 2023;14
https://doi.org/10.3389/fimmu.2023.1087397 |
| 77 | Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, Wu P, Shi Y, Mao F, Yan Y, Xu W, Qian H: Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano 2018;12:7613-7628.
https://doi.org/10.1021/acsnano.7b07643 |
| 78 | Caglar GS, Ozdemir ED, Cengiz SD, Demirtaş S: Sex‐hormone‐binding globulin early in pregnancy for the prediction of severe gestational diabetes mellitus and related complications. Journal of Obstetrics and Gynaecology Research 2012;38:1286-1293.
https://doi.org/10.1111/j.1447-0756.2012.01870.x |
| 79 | Richardson A, Carpenter M: Inflammatory mediators in gestational diabetes mellitus. Obstetrics and Gynecology Clinics of North America 2007;34:213-224.
https://doi.org/10.1016/j.ogc.2007.04.001 |
| 80 | Karacay O, Sepici-Dincel A, Karcaaltincaba D, Sahin D, Yalvaç S, Akyol M, Kandemir O, Altan N: A quantitative evaluation of total antioxidant status and oxidative stress markers in preeclampsia and gestational diabetic patients in 24-36 weeks of gestation. Diabetes Research and Clinical Practice 2010;89:231-238.
https://doi.org/10.1016/j.diabres.2010.04.015 |
| 81 | Feng S, Zhang Z, Xu S, Mao X, Feng Y, Zhu Y, Liu C: The Prevalence of Thyroid Nodules and Their Association with Metabolic Syndrome Risk Factors in a Moderate Iodine Intake Area. Metabolic Syndrome and Related Disorders 2016;15:93-97.
https://doi.org/10.1089/met.2016.0077 |
| 82 | Jones M, Defever E, Letsinger A, Steele J, Mackintosh KA: A mixed-studies systematic review and meta-analysis of school-based interventions to promote physical activity and/or reduce sedentary time in children. Journal of Sport and Health Science 2020;9:3-17.
https://doi.org/10.1016/j.jshs.2019.06.009 |
| 83 | Abd El-Hakim YM, Abdel-Rahman Mohamed A, Khater SI, Hamed Arisha A, Metwally MMM, Nassan MA, Hassan ME: Chitosan-Stabilized Selenium Nanoparticles and Metformin Synergistically Rescue Testicular Oxidative Damage and Steroidogenesis-Related Genes Dysregulation in High-Fat Diet/Streptozotocin-Induced Diabetic Rats. Antioxidants. DOI: 10.3390/antiox10010017.
https://doi.org/10.3390/antiox10010017 |
| 84 | Lee K, Paulino Silvestre M, Raben A, Fogelholm M, Poppitt S: Serum IGF-II and IGF2R in diabetes and obesity: relation to weight, fasting glucose and weight loss intervention in an overweight, pre-diabetic NZ population: ECO2017 24th European Congress of Obesity, S. Karger AG, 2017,
|
| 85 | Li B-Q, Liu X-Y, Mao T, Zheng T-H, Zhang P, Zhang Q, Zhang Y, Li X-Y: The research progress of anti-inflammatory and anti-fibrosis treatment of chronic pancreatitis. Frontiers in Oncology 2022;12
https://doi.org/10.3389/fonc.2022.1050274 |
| 86 | Tesone M, Ladenheim RG, Charreau EH: Alterations in the prolactin secretion in streptozotocin-induced diabetic rats. Correlation with pituitary and hypothalamus estradiol receptors. Molecular and Cellular Endocrinology 1985;43:135-140.
https://doi.org/10.1016/0303-7207(85)90076-0 |
| 87 | Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Méneur C, Bernal-Mizrachi E: Natural history of β-cell adaptation and failure in type 2 diabetes. Molecular Aspects of Medicine 2015;42:19-41.
https://doi.org/10.1016/j.mam.2014.12.002 |
| 88 | Alipio Z, Liao W, Roemer EJ, Waner M, Fink LM, Ward DC, Ma Y: Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic β-like cells. Proceedings of the National Academy of Sciences 2010;107:13426-13431.
https://doi.org/10.1073/pnas.1007884107 |
| 89 | Szunerits S, Melinte S, Barras A, Pagneux Q, Voronova A, Abderrahmani A, Boukherroub R: The impact of chemical engineering and technological advances on managing diabetes: present and future concepts. Chemical Society Reviews 2021;50:2102-2146.
https://doi.org/10.1039/C9CS00886A |
| 90 | AlZaim I, Hammoud SH, Al-Koussa H, Ghazi A, Eid AH, El-Yazbi AF: Adipose Tissue Immunomodulation: A Novel Therapeutic Approach in Cardiovascular and Metabolic Diseases. Frontiers in Cardiovascular Medicine 2020;7
https://doi.org/10.3389/fcvm.2020.602088 |
| 91 | Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank A-M, Bocian C, Woelk L, Fan H, Logan DW, Schürmann A: Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell stem cell 2017;20:771-784. .
https://doi.org/10.1016/j.stem.2017.02.009 |
| 92 | Mills EG, Yang L, Nielsen MF, Kassem M, Dhillo WS, Comninos AN: The Relationship Between Bone and Reproductive Hormones Beyond Estrogens and Androgens. Endocrine Reviews 2021;42:691-719.
https://doi.org/10.1210/endrev/bnab015 |
| 93 | Putra A, Suwiryo ZH, Muhar AM, Widyatmoko A, Rahmi FL: The role of mesenchymal stem cells in regulating PDGF and VEGF during pancreatic islet cells regeneration in diabetic animal model. Folia Medica 2021;63:875-883.
https://doi.org/10.3897/folmed.63.e57636 |
| 94 | Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P: Mesenchymal Stem Cells Protect Breast Cancer Cells through Regulatory T Cells: Role of Mesenchymal Stem Cell-Derived TGF-β. The Journal of Immunology 2010;184:5885-5894.
https://doi.org/10.4049/jimmunol.0903143 |
| 95 | Alsharif KF, Hamad AA, Alblihd MA, Ali FAZ, Mohammed SA, Theyab A, Al-Amer OM, Almuqati MS, Almalki AA, Albarakati AJA, Alzahrani KJ, Albrakati A, Albarakati MH, Abass D, Lokman MS, Elmahallawy EK: Melatonin downregulates the increased hepatic alpha-fetoprotein expression and restores pancreatic beta cells in a streptozotocin-induced diabetic rat model: a clinical, biochemical, immunohistochemical, and descriptive histopathological study. Frontiers in veterinary science 2023;10:1214533.
https://doi.org/10.3389/fvets.2023.1214533 |
| 96 | Dai X-Q, Camunas-Soler J, Briant LJ, Dos Santos T, Spigelman AF, Walker EM, e Drigo RA, Bautista A, Jones RC, Avrahami D: Heterogenous impairment of α cell function in type 2 diabetes is linked to cell maturation state. Cell Metabolism 2022;34:256-268. e255.
https://doi.org/10.1016/j.cmet.2021.12.021 |
| 97 | Lee S, Xu H, Van Vleck A, Mawla AM, Li AM, Ye J, Huising MO, Annes JP: β-Cell Succinate Dehydrogenase Deficiency Triggers Metabolic Dysfunction and Insulinopenic Diabetes. Diabetes 2022;71:1439-1453.
https://doi.org/10.2337/db21-0834 |
| 98 | Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B: Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovascular Research 2019;115:1205-1216.
https://doi.org/10.1093/cvr/cvz040 |
| 99 | Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41-46.
https://doi.org/10.1038/nature02520 |
| 100 | Egozi A, Llivichuzhca-Loja D, McCourt BT, Bahar Halpern K, Farack L, An X, Wang F, Chen K, Konnikova L, Itzkovitz S: Insulin is expressed by enteroendocrine cells during human fetal development. Nature Medicine 2021;27:2104-2107.
https://doi.org/10.1038/s41591-021-01586-1 |
| 101 | Wang HL, Wei B, He HJ, Huang XR, Sheng JY, Chen XC, Wang L, Tan RZ, Li JC, Liu J, Yang SJ, Ma RC, Lan HY: Smad3 deficiency improves islet-based therapy for diabetes and diabetic kidney injury by promoting β cell proliferation via the E2F3-dependent mechanism. Theranostics 2022;12:379-395.
https://doi.org/10.7150/thno.67034 |
| 102 | Sirajudeen S, Shah I, Karam SM, Al Menhali A: Seven-Month Vitamin D Deficiency Inhibits Gastric Epithelial Cell Proliferation, Stimulates Acid Secretion, and Differentially Alters Cell Lineages in the Gastric Glands. Nutrients. DOI: 10.3390/nu15214648.
https://doi.org/10.3390/nu15214648 |
| 103 | Wang D, Kuang Y, Wan Z, Li P, Zhao J, Zhu H, Liu Y: Aspartate Alleviates Colonic Epithelial Damage by Regulating Intestinal Stem Cell Proliferation and Differentiation via Mitochondrial Dynamics. Molecular Nutrition & Food Research 2022;66:2200168.
https://doi.org/10.1002/mnfr.202200168 |