Original Article - DOI:10.33594/000000745
Accepted 3 December 2024 - Published online 13 December 2024
Co-Treatment with Cranberry and Vitamin-C Mitigates Reproductive Toxicities Induced by Phenobarbital in Male Rats
bDepartment of Plant Biotechnology , Biotechnology Research Center, Al-Nahrain University, Baghdad, Iraq,
cPolymer Research Unit, College of Science, Mustansiriyah University, Baghdad, Iraq,
dMarine Biology Department, Faculty of Marine Sciences, King Abdulaziz University, Saudi Arabia,
eDepartment of Biology, College of Science, Taibah University, Madinah, Saudi Arabia, P.Obox 42356,
fZoology Department, Faculty of Science, Tanta University, Tanta, Egypt
Keywords
Abstract
Background/Aims:
Phenobarbital (PB), commonly used for epilepsy management, is associated with testicular dysfunction after prolonged use. This study aimed to evaluate the ameliorative effects of cranberry (CB) and vitamin C (Vit-C) on PB-induced reproductive toxicity in rats.Methods:
Forty male Wistar rats were divided into five groups. G1 was the negative control, while G2 received PB (160 mg/kg orally) for one month. Groups G3 and G4 received PB followed by CB (500 mg/kg) and Vit-C (27 mg/kg) treatments, respectively. G5 received PB followed by a combined CB and Vit-C regimen. The levels of catalase (CAT), superoxide dismutase (SOD), glutathione reduced (GSH), and malondialdehyde (MDA) were determined using standard biochemical assays. Histological changes in testicular tissues were assessed, and caspase-3 expression was quantified via immunohistochemistry.Results:
PB exposure increased MDA levels, reduced SOD and CAT activity, and disrupted testicular histology, with elevated caspase-3 expression indicating heightened apoptosis. Treatment with CB or Vit-C significantly restored antioxidant enzyme activities, reduced MDA levels, and ameliorated histological abnormalities. Co-treatment with CB and Vit-C yielded the most pronounced protective effects, including reduced caspase-3 expression and improved testicular structure.Conclusion:
CB and Vit-C demonstrate significant protective effects against PB-induced testicular toxicity, likely due to their antioxidative and anti-apoptotic properties. Co-administration of these agents offers an effective strategy to mitigate reproductive toxicities associated with prolonged PB use.Introduction
Male reproductive well-being is equivalent to overall wellness, as it can directly or indirectly affect fertility and the integrity of semen. A lot of pharmaceutical drugs have been discovered to influence male reproductive efficacy adversely. Phenobarbital (Pb) is a pharmacological compound recommended by the World Health Organization (WHO) for treating specific types of epilepsy. The application of this therapeutic strategy is commonly noticed in the medical management of seizures among pediatric populations (WHO, 2013). As discussed in previous studies, the injected form has been utilized to control status epilepticus (Brodie & Kwan, 2012; Falsaperla et al., 2019; Lewis & Adams, 2022). Rahim et al. (2021) reported that PB occasionally treats insomnia, anxiety disorder`s, pharmaceutical withdrawal signs and symptoms, and perioperative assistance.
Researchers worldwide have shown considerable interest in using herbal remedies in illness therapy. This interest stems from the possible medicinal advantages of regulating numerous targets and signalling cascades. As a result, natural product mixtures have emerged as a predominant reservoir of therapeutics readily accessible in the commercial market (Newman & Cragg, 2020; Xueni et al., 2022). These advantages include a high tolerance level, low toxicity, and the capacity to regulate many pharmacological targets. Moreover, it has been recognized that natural products exhibit significant potential for simultaneous incorporation with diverse medicinal products, hence offering a promising solution to the persistent obstacles presented by traditional medications (Sauter, 2020).
Cesonienė and Daubaras (2015) reported that cranberry (CB) is distinguished by its notable dietary fibre composition, organic acids, flavonoids, glycosides, terpenoids, tannins, and alkaloids. The above substances demonstrate robust antioxidant characteristics and substantial protective advantages over cancer, inflammation, and mutagenesis (Blumberg et al ., 2013; Ali et al ., 2014; Singh et al ., 2016). Fruits with a significant abundance of phenolic compounds have been identified as having high levels of these substances, hence being classified as CB. Stobnicka et al . (2018) asserted that these compounds possess numerous health benefits and have preventive properties for various illnesses. The investigations carried out by Hussain et al. (2017) and Kurpik et al . (2021) have indicated that the usage of CB-extract has demonstrated a favourable impact on the changes observed in blood oxidative stress indicators in rats that have been exposed to liver damage caused by CCl4. Prior work has demonstrated the possible beneficial effects of CB extract in reducing oxidative stress induced by eating diets that contain fat and cholesterol, as well as being overweight, in rats (Boušová et al ., 2015; Faheem et al ., 2020; Kurpik et al ., 2021).
Vitamin-C (Vit-C), scientifically known as ascorbic acid, is categorized as a water-soluble vitamin. This characteristic allows it to efficiently neutralize aqueous radicals that possess the capacity to harm membrane lipids (Padayatty & Levine, 2016). Vit-C is involved in many physiological activities, encompassing the production of collagen, carnitine, and hormones, alongside the regulation of translation (Dosed et al ., 2021). Furthermore, previous research has indicated that the antioxidative power contributes to the advancement of spermatogenesis, enhancement of the quality of semen, and improvement of fecundity (Vijayprasad, 2014).
An observed deficiency in Vit-C has been found to induce oxidative stress in the testes, thereby interfering with the mechanisms involved in testosterone synthesis and spermatogenesis. In recent years, there has been a notable surge in the usage of supplements containing Vit-C in the dietary regimens of rats, specifically targeting the enhancement of their production and reproductive capacities. A study conducted by Sanghishetti et al. (2014) revealed that the inclusion of Vit-C in an individual’s dietary intake has positively impacted the augmentation of sperm production and concentration. The study aimed to evaluate the ameliorative effect of the co-treatment with cranberry and Vit-C on the reproductive toxicity that was induced by phenobarbital in experimental rats.
Materials and Methods
Chemicals
Sigma Company, an American-based organization, was a Phenobarbital (PB) supplier. Cranberry (CB) and Vitamin-C (Vit-C) were acquired from a pharmaceutical establishment in Cairo, Egypt. The Bio-diagnostic Company was the source of the phosphate buffer saline (PBS), as well as kits for superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), and glutathione reduced (GSH). The primary antibody targeting caspase-3 was acquired from Novacastra Laboratories Ltd., a company based in the United Kingdom.
Experimental animals
The study utilized adult male Wistar rats sourced from the National Research Center (NRC) in Cairo, Egypt. These rats had an average weight of 120±4 g. Animals and the experimental design for this study were authorized by the institutional animal care committee of the Faculty of Science at Tanta University in Egypt, under reference number 0139. Before being separated into groups, male rats were subjected to a one-week acclimation period under the animal house conditions at the Zoology Department, Faculty of Science, Cairo University. The desired temperature and relative humidity values were 22 ± 1 ºC and 55 ± 5%, respectively. The light-dark cycle was successfully applied to simulate day and night conditions. Rats were provided unrestricted access to tap water for drinking and consuming a standard pelleted animal diet.
Experimental protocol
Forty rats were divided into five groups, each consisting of eight based on their body weights. This categorization was carried out to reduce the individual variation between groups. G1 served as a negative control. G2 was administered orally with PB (160 mg/kg) for a month. G3 and G4 were administered with PB as in G2, then treated daily for a month with CB (500 mg/kg) and Vit-C (27mg/kg) by oral gavage. G5 was administered with PB in G2 and CB/Vit-C in G3 and G4.
Process of collecting samples
Testicular specimens from all rats were taken. Then, tissues were homogenized in a 5-10 ml cold buffer solution containing 50 mM potassium phosphate (pH 7.4), 1 mM EDTA, and 1 mL/L Triton X-100 per gram of tissue. The samples should be centrifugated at 4, 000 revolutions per minute for 15 minutes while maintaining a temperature of 4 degrees Celsius. The supernatant should be carefully removed for subsequent analysis and promptly placed in a chilled environment.
Assessment of superoxide dismutase activity
The methodology employed in this study is predicated on the enzyme’s capacity to impede the reduction of nitroblue tetrazolium dye mediated by phenazine methosulphate, as described by Nishikimi et al . (1972).
Determination of catalase activity
The catalase assay was performed using the protocol outlined by Aebi (1984). The enzyme catalase reacts with a predetermined amount of hydrogen peroxide (H2O2). After one minute, the catalase inhibitor carefully stops the reaction.
Quantification of reduced glutathione concentration
The assay for reduced glutathione was conducted using the methodology outlined by Beutler et al . (1963). The experimental approach involves utilizing the reduction reaction between 5, 5`-dithiobis (2-nitrobenzoic acid) (DTNB) and glutathione (GSH) to generate a molecule exhibiting a yellow colouration. The concentration of reduced chromogen is directly proportional to the concentration of GSH, and its absorbance may be quantified at a wavelength of 405 nm.
Quantification of malondialdehyde concentration
The quantification of lipid peroxides, namely as thiobarbituric acid reactive substances (TBARS), was conducted using the methodology outlined by Esterbauer and Cheeseman in 1990. In the experimental procedure involving the TBA test reactions, it was observed that the reaction between one molecule of malondialdehyde (MDA) and two molecules of thiobarbituric acid (TBA) resulted in the formation of a pink pigment. This pigment exhibited a maximum absorbance at a wavelength of 532 nm.
Sperm analysis
The methodology employed to ascertain the overall sperm count in the caudal epididymis closely resembled the procedure outlined by Robb et al . (1978). The fluid from the epididymis was expressed onto a petri dish containing 0.5 ml of physiological saline solution, maintained at a temperature of 37 ºC. A volume of 0.5 ml of the sperm suspension was introduced into 1 ml of the semen-diluting solution, followed by thorough mixing. A single droplet of sperm suspension was introduced into the chamber of a hemocytometer, and the spermatozoa were permitted to sediment by placing the hemocytometer in a humid environment for 10 minutes. The spermatozoa count in the hemocytometer squares was determined using a CX41 microscope at a magnification of 10X. The term “sperm concentration” refers to the number of spermatozoa present in each millilitre of epididymis plasma. The formula for calculating sperm count is determined by multiplying the number of sperm by the dilution factor and the depth factor and then dividing the result by the total number.
Motility of sperm cells
The progressive motility percentage was assessed using a light microscope, employing the methodology outlined by Slott et al. (1991). In this experimental procedure, a slide was carefully positioned onto the microscope stage and then subjected to a controlled temperature of 37 °C, achieved by utilizing a hot plate. A minute quantity of liquid semen was deposited onto a heated slide. The assessment of motility percentage was conducted at a magnification of 100×. Estimations were managed on three drops from each sample.
Viability of sperm cells
An aliquot of sperm suspension (10 µL) was applied to a slide, spread with a second slide, and thereafter permitted to dry. The dried samples were stained using a solution of 1% Eosin and 10% Nigrosin. The dye will permeate the cytoplasm of the sperm if the membrane has been damaged, a common occurrence in deceased or deteriorating spermatozoa. A minimum of 100 spermatozoa were evaluated using a CX41 microscope at a magnification of 400X to determine sperm viability (Morakinyo et al., 2009).
Histopathological examinations
The testes from various groups were subjected to fixation for histological investigation using light microscopy. The fixation process involved immersing testes in a 10% formal saline solution for twenty-four hours. The process of washing involved the use of tap water, followed by the application of ascending dilutions of alcohol for dehydration. Specimens underwent a clearing process using xylene. They were embedded in paraffin wax at a temperature of 56 degrees Celsius in an oven for twenty-four hours. Paraffin-embedded tissue blocks were prepared for sectioning using a 4-5 µ thickness microtome. Tissue sections were carefully uploaded onto glass slides, followed by deparaffinization. Subsequently, a hematoxylin and eosin stain was applied to enable routine histological examination, as described by Bancroft and Gamble (2002).
Immunohistochemistry (IHC) technique
Tissue sections were dewaxed and rehydrated, and 10% H2O2 was added for endogenous peroxidase blocking. Then, boiling with citrate buffer (pH 6.0) for antigen retrieval was performed. Sections were then incubated with the primary antibodies: anti-caspase 3 (BIOCYC GmbH, Germany). Subsequently, incubation with the secondary antibody, a biotinylated goat anti-rabbit immunoglobulin and avidin-biotin complex was carried out. The localization sites for caspase-3 in the cerebellar tissue were visualized by adding diaminobenzidine HCl, which was converted into a brown precipitate by peroxidase. Finally, Mayer’s hematoxylin was utilized for counterstaining (Bancroft & Stonard, 2013). The study of caspase-3 was conducted quantitatively utilizing ImageJ software (Version 1.53i). The DAB signal was quantified using ImageJ software to assess variations in immunoreactivity. Fifteen fields were chosen, each with five and three rats per group. The optical density was determined using the equation OD = log (Maximum grey intensity / mean grey intensity) to assess the level of immunoreactivity (darkness) exhibited by stained cells through the DAB signal, as described by Abd-Elhafeez et al . (2021).
Protein structure preparation
The protein structures for SOD (UniProt ID: P07895), THAS (UniProt ID: P49430), GSHB (UniProt ID: P46413) and CASP3 (UniProt ID: P55213) were obtained from their respective sources. The active sites of these proteins were predicted using the CB-Dock2 server [Yang Liu et al. 2022], which employs deep learning algorithms to identify potential binding pockets and catalytic sites.
Protein preparation for docking
The prepared protein structures were further processed using AutoDock Tools 1.5.7 (Morris et al., 2009). This involved adding polar hydrogen atoms, assigning Gasteiger charges, and merging non-polar hydrogen atoms. The resulting PDBQT files were used as input for molecular docking simulations.
Ligand preparation
Active constitute obtained from) Nemzer et al., 2022). The ligand molecules were retrieved from the PubChem database in their respective SDF formats. These ligands were then minimized using the Avogadro 1.2.0 software (Marcus et al., 2012), employing the Force Field (MMFF94) (Hanwell et al., 2012) and the Conjugate Gradients algorithm. The minimized ligand structures were converted to the PDBQT format compatible with AutoDock Vina (Trott et al., 2010).
Molecular docking
Molecular docking simulations were performed using AutoDock Vina (Trott et al., 2010), a widely used open-source molecular docking and virtual screening program. The prepared protein and ligand structures in PDBQT format were input for the docking simulations. The search space for the docking simulations was defined based on the predicted active site regions obtained from the cb-dock2 server.
Visualization and analysis
The docking results were visualized and analyzed using the BIOVIA Discovery Studio 2020 [BIOVIA 2020]. This software was used to inspect the protein-ligand interactions, calculate binding energies, and generate visual representations of the docked complexes.
Statistical analysis
The findings presented in this study represent the average values obtained from three independent replicates. The data were reported as the mean value plus or minus the standard deviation. The comparison between groups was conducted using a one-way analysis of variance (ANOVA). A Tukey post hoc analysis was performed to compare several groups and estimate the presence of a substantial disparity between means. In the context of statistical tests, a P value less than 0.05 was deemed statistically significant. The data and statistical analysis were conducted utilizing SPSS version 25.
Results
Treatment with CB and/ or Vit. C ameliorated PB–induced testicular oxidative stress toxicity in rats
The study indicated that administering CB and/or Vit-C (vitamins) as treatment yielded specific outcomes. The study investigated the ameliorative potential of CB/Vit-C on testicular oxidative stress toxicity produced by PB in rats. The findings revealed that there were no statistically significant alterations observed in the activities of testicular SOD and CAT, as well as the levels of GSH and MDA, among the control groups. In comparison to the control group, rats that were exposed to PB exhibited a statistically significant reduction (p < 0.05) in testicular SOD and CAT activity, as seen in Table 1. Moreover, rats exposed to PB exhibited a noteworthy reduction in the testicular GSH level and a substantial elevation (p < 0.05) in testicular MDA level compared to the control group. Rats injected with PB and subsequently treated with CB and/or Vit-C exhibited a substantial elevation in testicular SOD and CAT activities and a notable increase in testicular GSH levels. Conversely, there was a significant reduction (p < 0.05) in testicular MDA levels compared to rats treated solely with PB (Table 1).
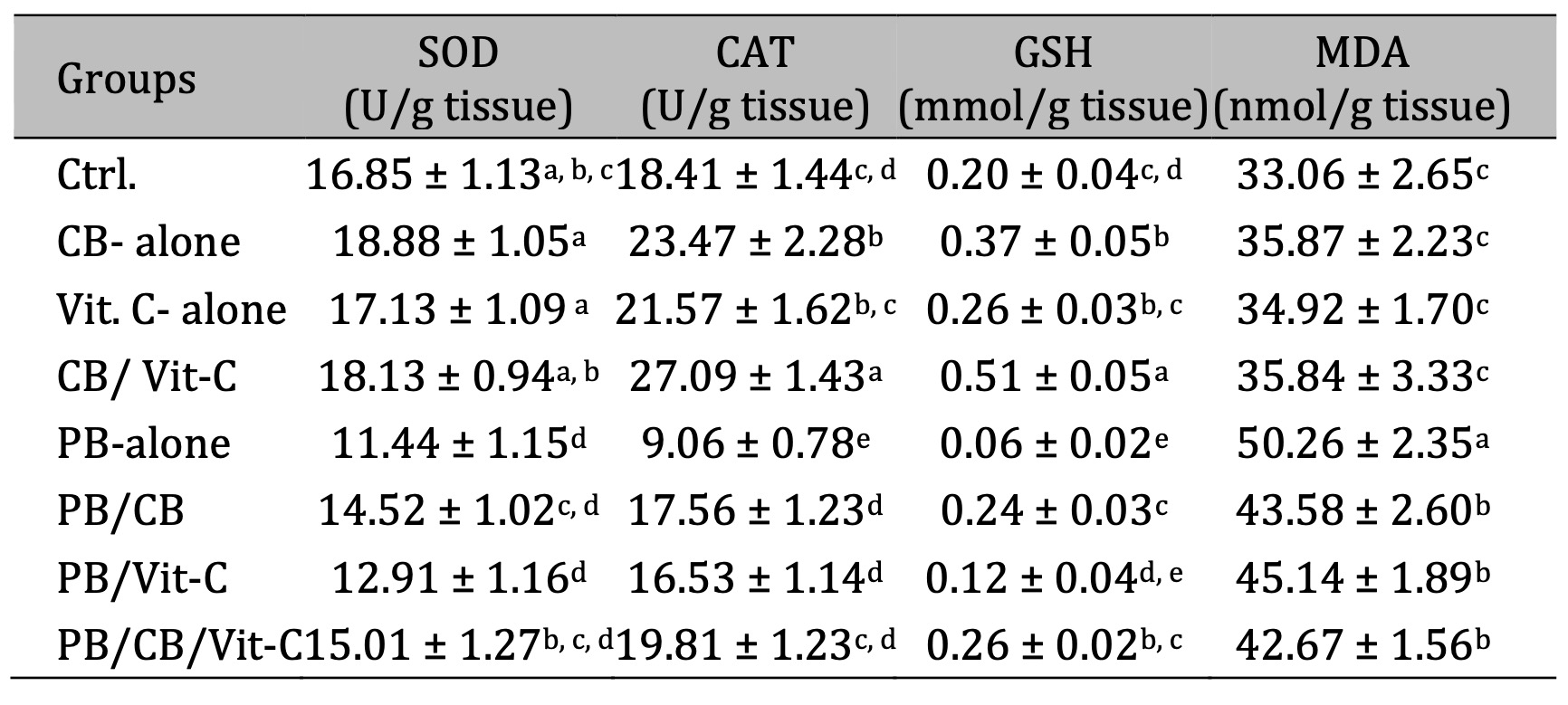
Table 1: Oxidative stress biomarkers of testicular tissue in the different groups. The values represented mean ± SD; SOD: Superoxide dismutase; CAT: Catalase; GSH: Reduced glutathione; MDA: Malondialdehyde; Ctrl: Control; PB: Phenobarbital; CB: cranberry; Vit-C: Vitamin C. P value < 0.05 was considered to be statistically significant. Means that do not share a letter are significantly different
Treatment with CB and/ or Vit. C mitigated PB-induced sperm dysfunction
The study’s findings indicated that administration of PB to a group of rats resulted in a statistically significant reduction in sperm count, motility, and vitality compared to the control group (p < 0.05). However, the administration of CB or Vit-C did not provide any statistically significant alterations in sperm count, motility, and vitality in comparison to the control group. The concurrent treatment of CB and Vit-C led to a notable enhancement in sperm count, motility, and vitality compared to rats administrated with PB (p < 0.05), as observed in (Table 2).
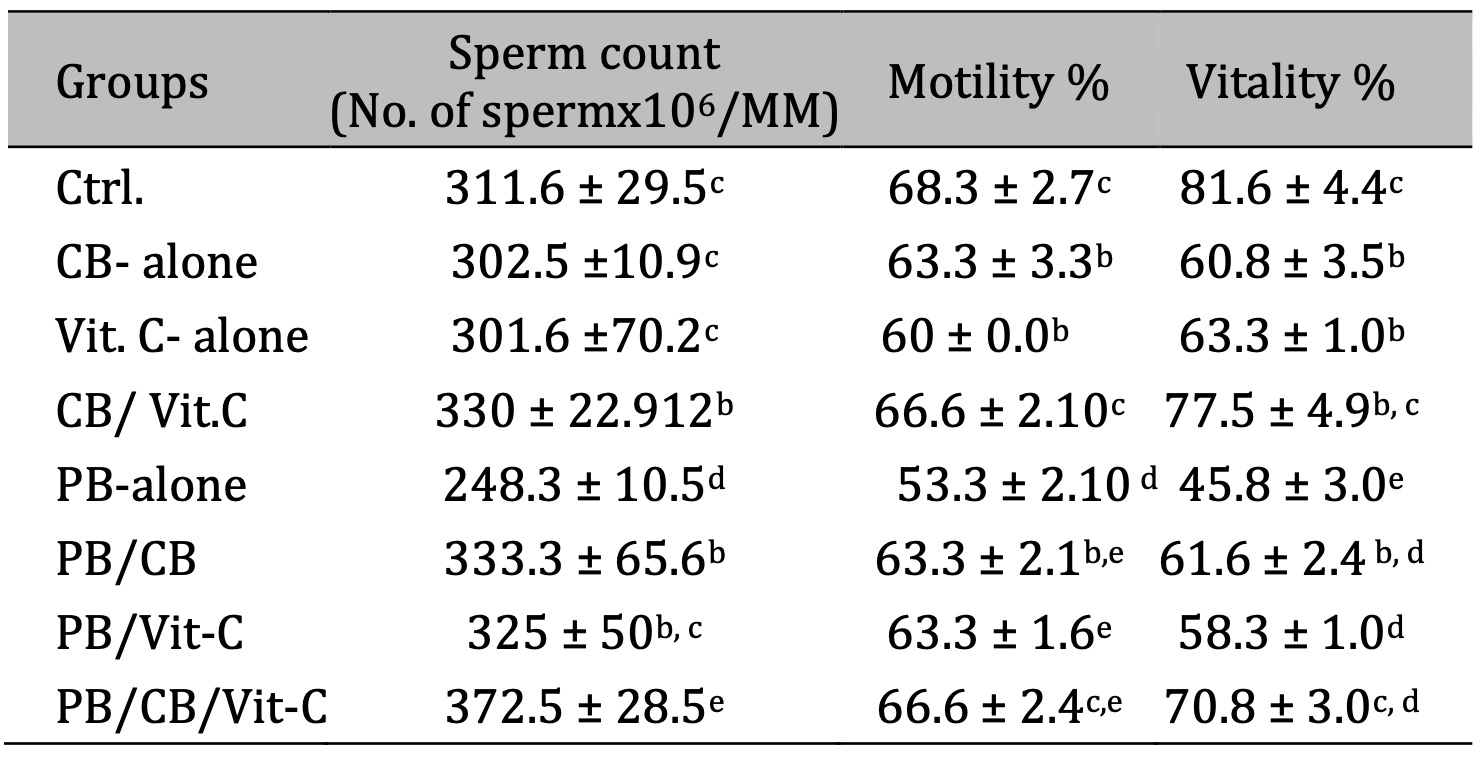
Table 2: The sperm analysis in different groups. The values represented mean ± SD; Ctrl: Control; PB: Phenobarbital; CB: cranberry; Vit-C: vitamin C. P value < 0.05 was statistically significant. Means that do not share a letter are significantly different
Treatment with CB and Vit-C ameliorated the histopathological changes in testicular tissue induced by PB
The analysis of control testis sections revealed the presence of seminiferous tubules and interstitial tissue exhibiting a regular structural arrangement. The interior of each tubule was covered by a stratified epithelium composed of spermatogenic cells. Spermatogenic cells include spermatogonia, spermatocytes, spermatids, and spermatozoa, all associated with healthy reproductive function. Interstitial cells (Leydig cells) could be observed (Fig. 1). Comparably, the tests of rats treated with CB and Vit-C exhibited a histological pattern nearly similar to that of the control group (Fig. 1).
Furthermore, the oral gavage of PB (160 mg/kg for one month) revealed several histopathological lesions; some seminiferous tubules appeared empty from spermatozoa, and one appeared ruptured. There was a notable decline in the lineage of spermatogenic cells. The basement membrane showed a dissimilar and undulating structure. Some damaged spermatogenic cells appeared detached in the luminal space of specific seminiferous tubules. Within the intratubular connective tissue, an absence of spermatozoa was seen within the lumen. Figure (2) showed an interstitial blood artery exhibiting congestion concurrently with the absence of germinal epithelium. Rats treated with CB after exposure to PB exhibited seminiferous tubules and interstitial tissue with histological criteria similar to control ones. Figure (2) indicated a moderate loss of germinal epithelium series. After exposure to PB, rats treated with Vit-C exhibited seminiferous tubules that displayed complete spermatogenesis and an intact germinal epithelial series. Furthermore, the lumen of these tubules contains spermatozoa (Fig. 1).
The administration of CB and Vit-C following PB exposure in rats demonstrated a noticeable improvement. Typical visual characteristics of testicular tissue include the presence of seminiferous tubules exhibiting complete spermatogenesis and a continuous germinal epithelial series consisting of spermatogonia, spermatocytes, spermatids, and spermatozoa. A considerable quantity of spermatozoa was observed within the lumen. The inter-tubular gap was typical, including many intact interstitial cells (Fig. 2).
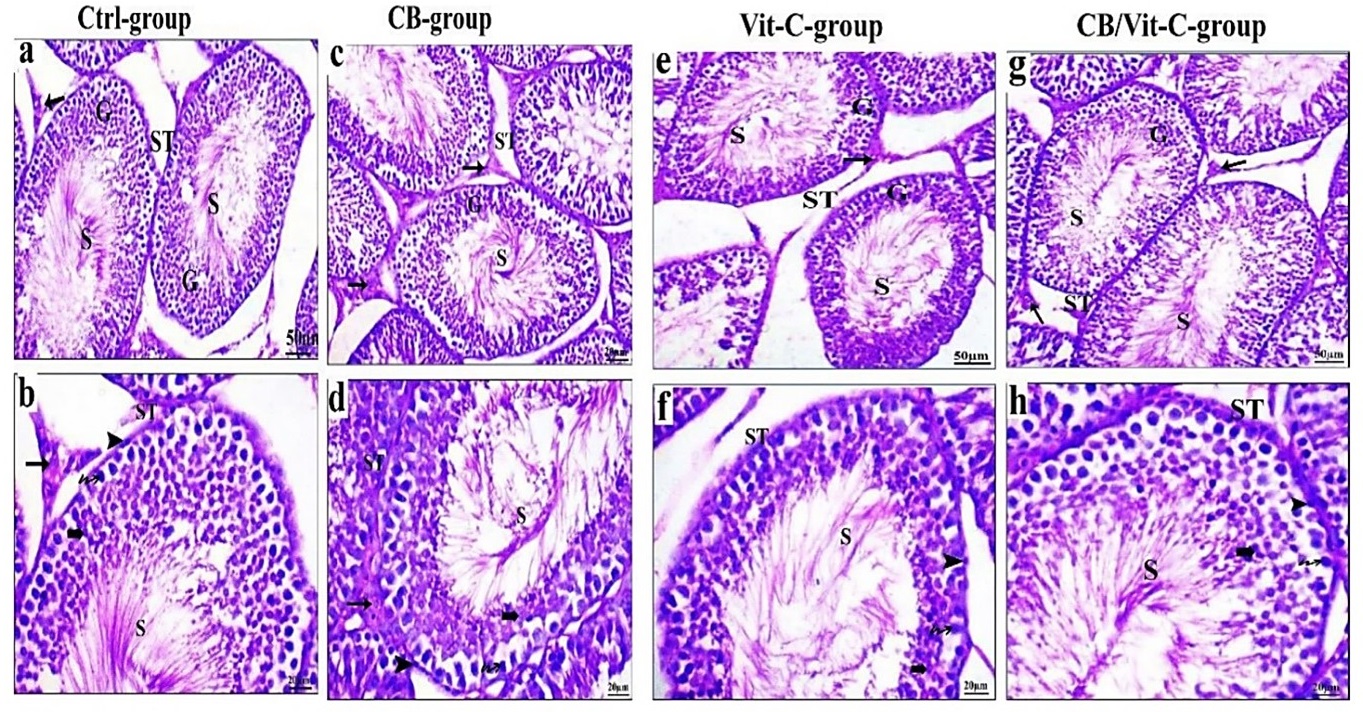
Fig. 1: Sections of testis of different treated groups showing the normal histological structure of seminiferous tubule (ST), sperm (S), interstitial cells (arrow), germinal epithelium (G), spermatocyte (Zigzag row), spermatid (´Bold arrow), spermatogonia (Head arrow). (H & E stain.
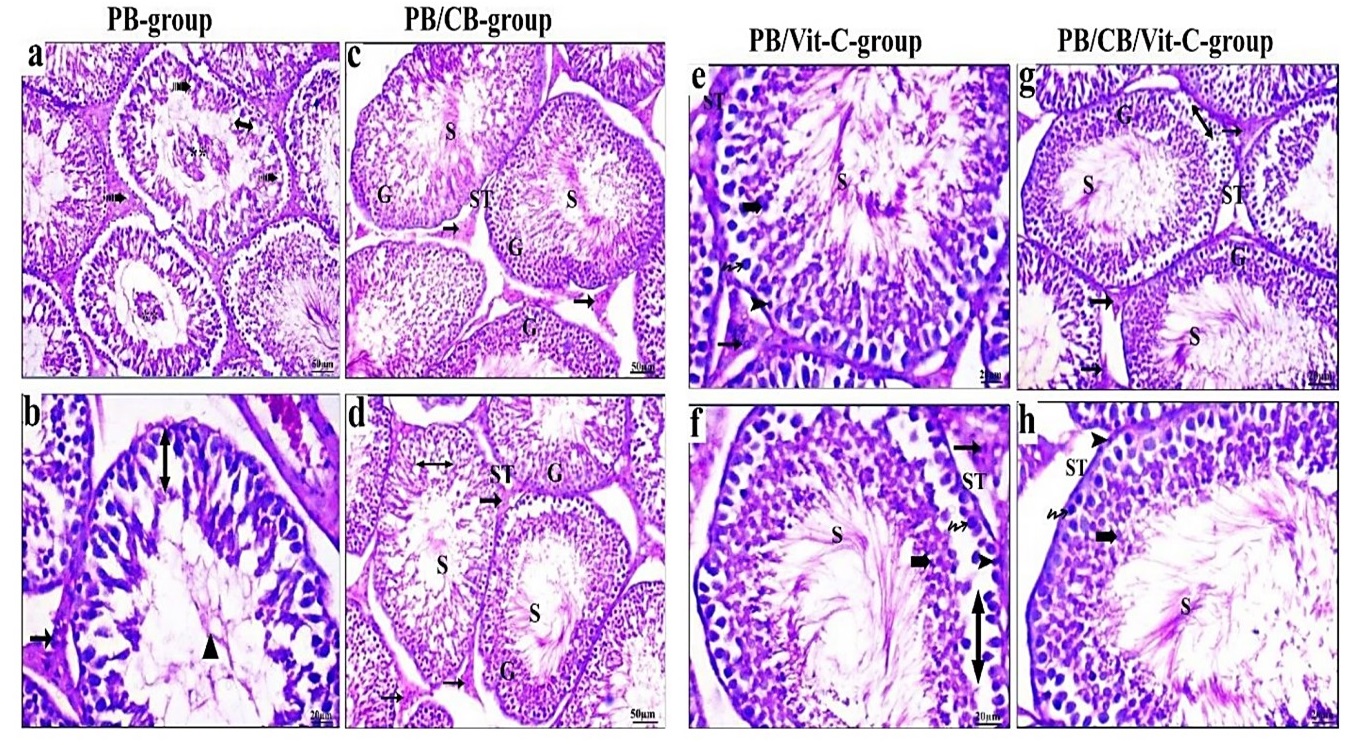
Fig. 2: H&E-stained sections of testes. Sections of testis of PB-treated rats showing lumen with damaged cells (**), loss of germinal epithelium series (double head arrow), vacuoles (dash arrow), lumen without spermatozoa (triangle). Treated groups after PB-injection showing maker improvement in testis structure of seminiferous tubule (ST), sperm (S), interstitial cells (arrow), germinal epithelium (G), and few losses of germinal epithelium series (double head arrow). Vit-C after PB- injection showing spermatocyte (Zigzag arrow), spermatid (Bold arrow), spermatogonia (Head arrow)- (H & E stain).
Effect of the CB or/and Vit-C treatment on immunohistochemistry for activated caspase-3 in testis tissues of PB-treated rats
Compared to the control group, the group that received PB exhibited a statistically significant elevation (p < 0.05) in the expression of caspase-3 in the tissue of the testes. The rats who received PB and were subsequently treated with either CB or Vit-C exhibited a notable reduction (p < 0.05) in the expression of immunohistochemistry caspase-3 compared to the rats that only received PB. In comparison to rats treated just with PB, the group of rats treated with both PB and co-administered with CB and Vit-C exhibited the most notable reduction (p < 0.05) in the expression of caspase-3 (Fig. 3&4).
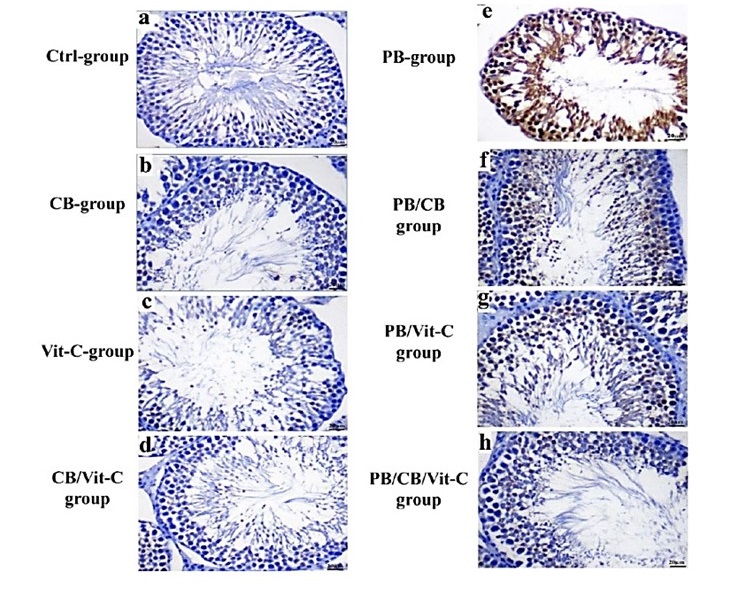
Fig. 3: Photomicrographs of section of immunohistochemistry comparing 4 staining in testis tissue from different experimental groups ( immunohistochemical staining with caspase 3).
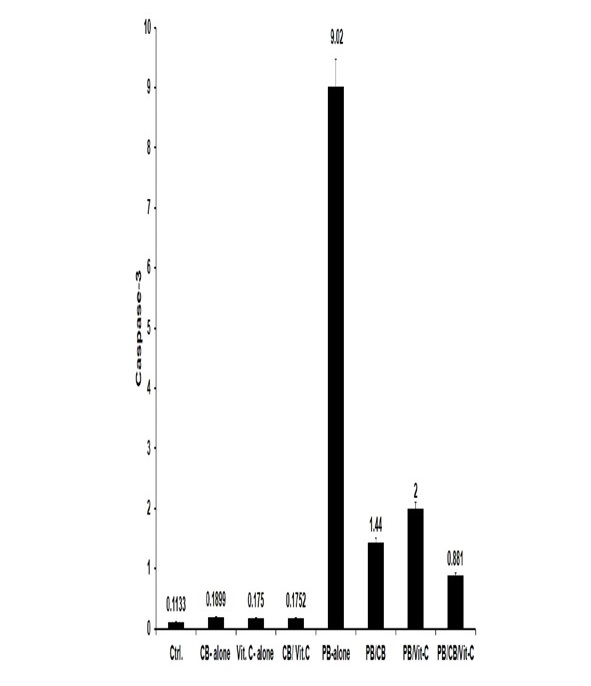
Fig. 4: Immunohistochemical caspase-3 expression in testis tissue. Crtl: Control; PB: Phenobarbital; CB: CB; Vit-C P value < 0.05 was considered to be statistically significant. Means that do not share a letter are significantly different.
Bioinformatics analysis
The comprehensive bioinformatics analysis revealed crucial insights into the molecular interactions between the studied compounds and target proteins involved in oxidative stress and apoptotic pathways. The binding energy calculations demonstrated that folate and riboflavin consistently exhibited the strongest interactions across all target proteins, suggesting their potential protective role against PB-induced toxicity. The analysis of interaction patterns revealed that most compounds form stable complexes through a sophisticated combination of hydrogen bonding, π-π interactions, and hydrophobic contacts.
Therefore, the molecular docking highlighted those proteins THAS and GSHB as the most favorable targets for most compounds, based on binding affinity patterns and interaction profiles. The presence of multiple hydrogen bonds and π-interactions in these complexes indicates stable binding modes that could be physiologically relevant. Particularly noteworthy was the strong binding affinity of folate, which correlates well with its potential protective effects observed in experimental studies.
The detailed molecular interactions of PB with CASP3 provided valuable structural insights into its mechanism of action in inducing apoptosis. So that, the computational findings provide strong support for the experimental observations and offer a molecular-level understanding of the protective mechanisms of CB and Vit-C against PB-induced reproductive toxicity. The analysis provides a structural basis for understanding how these compounds might interact with their target proteins to modulate their activity and potentially mitigate the toxic effects of PB.
Bioinformatics finding
As shown in Table 3, the data indicated that the binding energy (ΔG) values range from -4.2 to -7.8 kcal/mol across all compounds and proteins when the most negative value is the most favorable interaction. The results showed that Folate had the strongest binding affinity across all proteins, with particularly strong binding to THAS (-7.8 kcal/mol), especially with GSHB (-7.2 kcal/mol) Niacin generally shows the weakest binding affinities among all compounds and The target protein THAS appears to form more stable complexes with most compounds. The data represented in Table 4 showed the 3D and 2D interaction view for each affinity Phenobarbitals and proteins, in ribbon shaped proteins when (Red) for α-helix and blue for β-sheets in 3D interaction. Types of interactions extracted from 2D diagram while CASP3 Shows multiple π-π T-shaped interactions with tyrosine residues (TYR195, TYR197) and conventional hydrogen bonding however GSHB Exhibits strong hydrogen bonding network involving ARG215 and TYR270, plus hydrophobic interaction with ALA462. Then, SOD forms conventional hydrogen bonds with TRP210 and GLY110, with additional π-alkyl interaction with LYS114. Table 5 showed the interaction 3D and 2D for folate and each protein while 3D represents ribbon shaped protein with Red for α-Helix and Blue for β-Sheets. The interaction with CASP3 Shows extensive hydrogen bonding network involving multiple residues (LYS137, THR140, TYR195, TYR197) and with GSHB: Exhibits the most diverse interaction profile with attractive charges, conventional H-bonds, and π-sigma interactions , However, the interaction with SOD Forms multiple conventional hydrogen bonds and unique π-anion interaction with GLU211 and THAS Shows fewer but specific interactions including conventional H-bonds with ARG residues and π-sigma interactions
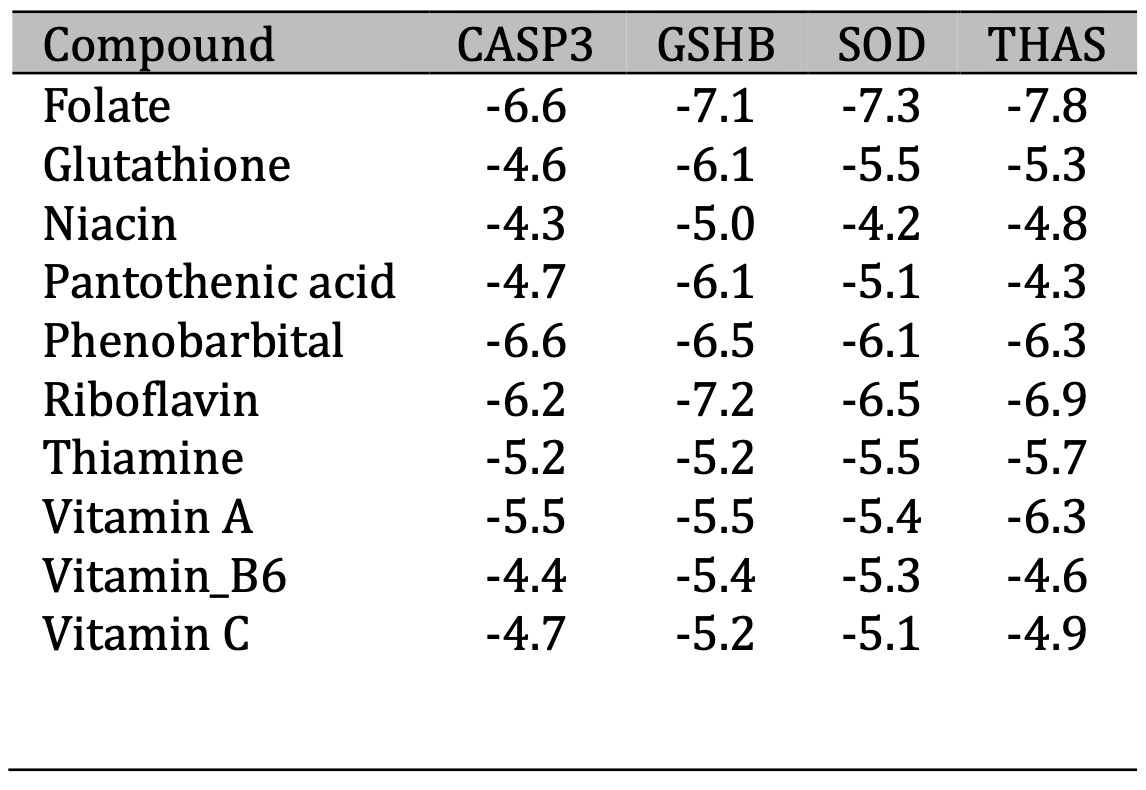
Table 3: ∆G (Kcal/mol) and binding affinity for each protein with active compounds
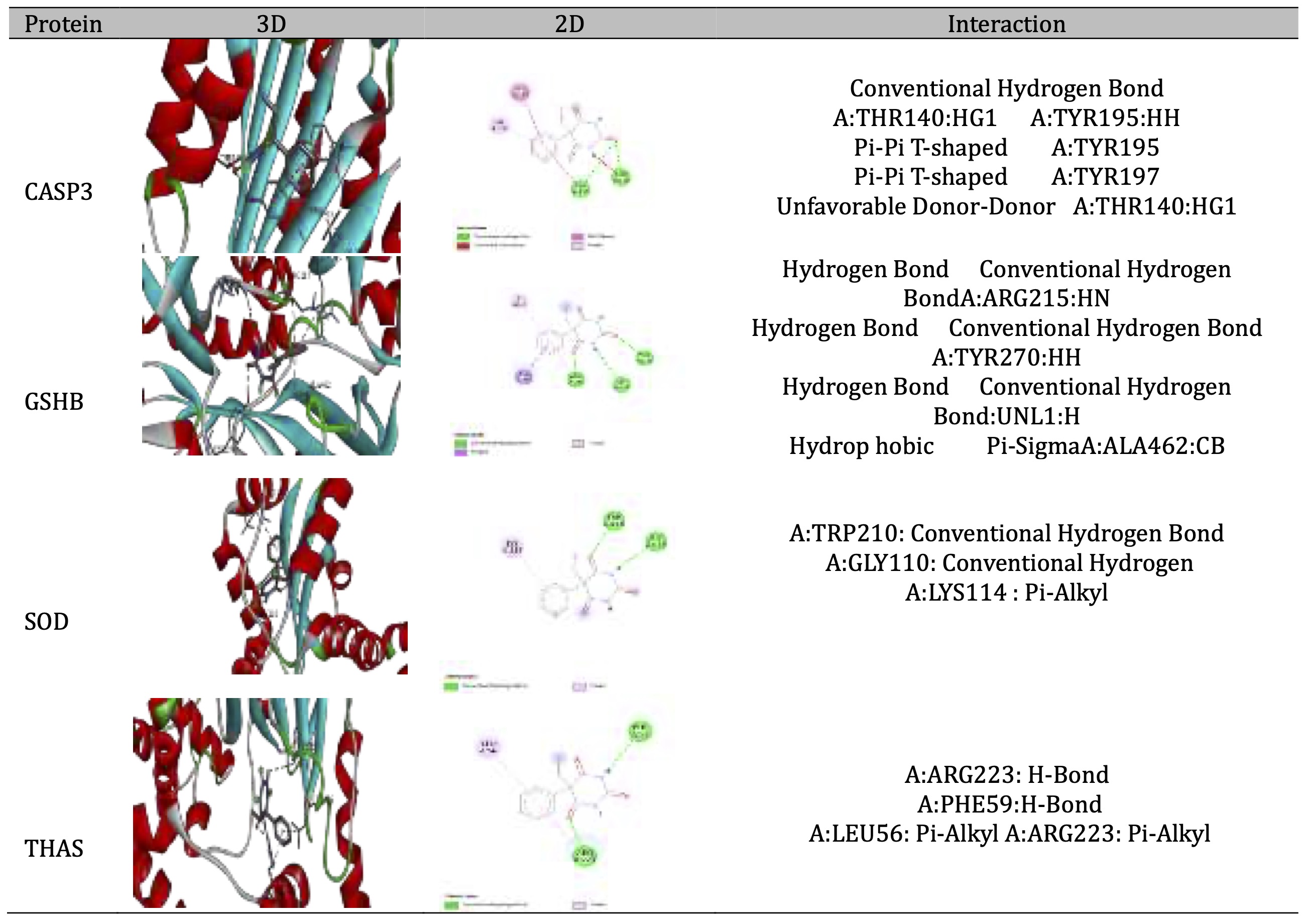
Table 4: 3D and 2D interaction between Phenobarbitals with different proteins
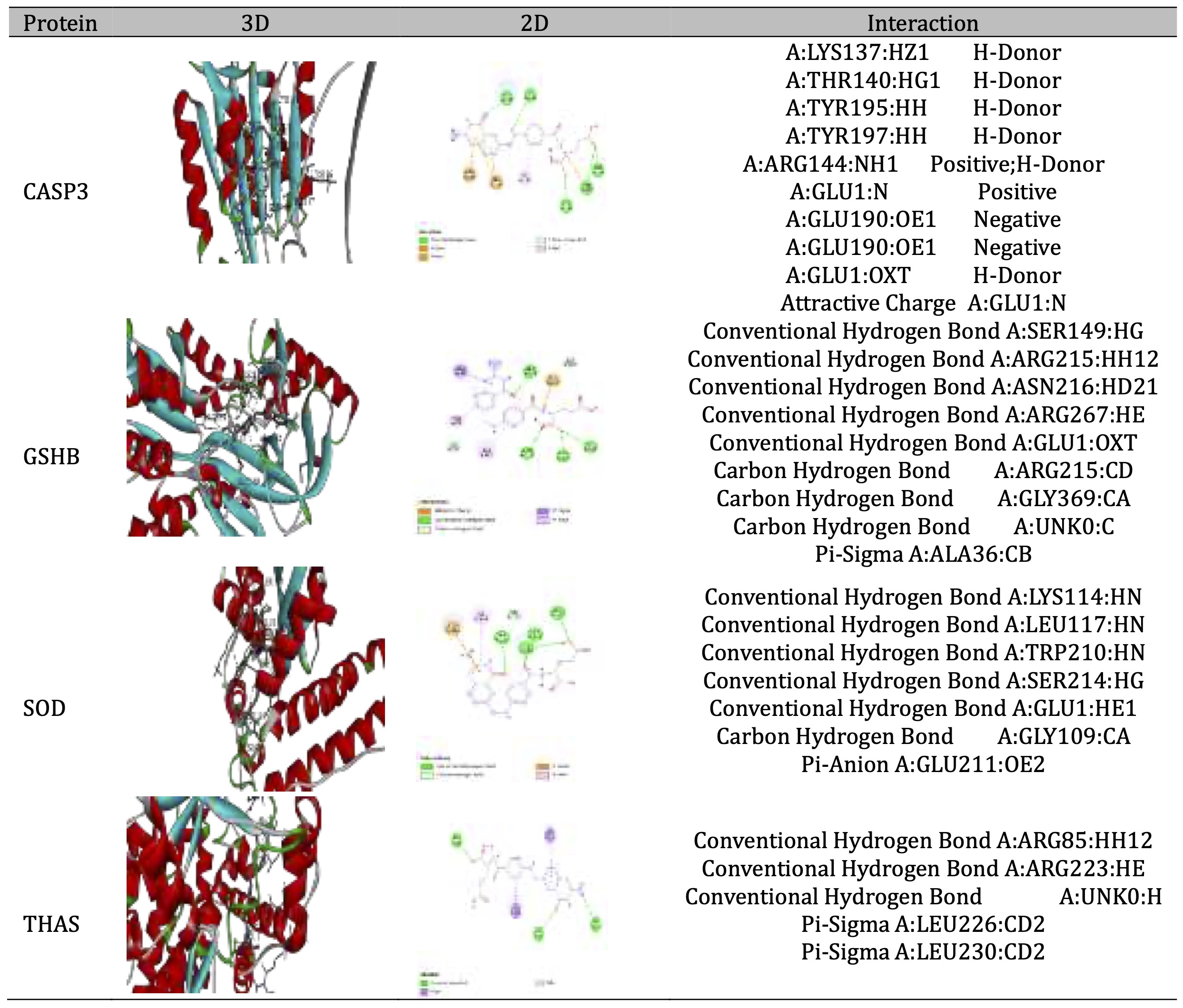
Table 5: 3D and 2D interaction between folate with different proteins
Discussion
Phenobarbital (PB) is a pharmacological agent widely utilized as an efficacious antiepileptic drug for the extended treatment of epilepsy. Nevertheless, it is crucial to note that specific negative consequences are linked to this phenomenon (Hermans et al., 2022). The CB is a botanical species classified under the Ericaceae family, renowned for its medicinal attributes. Based on the findings of Cesonienˇe et al. (2015), it has been observed that CB demonstrates increased levels of dietary fibre, organic acids, flavonoids, glycosides, terpenoids, tannins, and alkaloids. Ali et al. (2015) state that the compounds mentioned above demonstrate robust antioxidant characteristics and provide substantial protective benefits against cancer, inflammation, and mutagenesis.
Vit-C, scientifically called ascorbic acid, is a potent antioxidant abundantly found in fresh berries. This vitamin has potent reducing activities and is highly known for its notable antioxidant action. This result is accomplished by mitigating the deleterious impacts of free radicals and other reactive oxygen species arising from cellular metabolism. Sona Skrovankova et al. (2015) have shown a correlation between these reactive species and the occurrence of oxidative damage in different organs. This study aimed to investigate the potential ameliorative benefits of CB and/or Vit-C against reproductive damage induced by treatment with PB.
The results of this study indicate that the administration of PB at a dosage of 160 mg/kg for one month led to a significant increase in the relative proportions of testis weight. The observed outcome can be ascribed to the potential adverse effects of PB. The administration of Vit-C or CB decreased the observed increase in testis weight since these treatments demonstrated beneficial effects on the testicular tissue. Kurpik et al. (2021) conducted a study that revealed CB’s antioxidant properties, highlighting its potential to offer testicular protection. Sanghishetti et al. (2014) assert that Vit-C demonstrates antioxidant characteristics in the testis, hence playing a pivotal role in protecting testicular tissues from oxidative harm. Lapshina et al. (2015) reported that the flavonoids found in CB can effectively neutralize free radicals, superoxide radicals, hydroxyl radicals, and lipid peroxidation.
Furthermore, they could attenuate the detrimental effects of mitochondrial damage, such as fragmentation and loss of membrane integrity. Vit-C exhibits protective benefits due to its antioxidative characteristics. SOD and GSH are enzymatic entities identified as essential components in scavenging ROS. Reactive oxygen species have been observed to influence several cellular signalling pathways at low levels substantially. However, they can also induce cytotoxic effects by harming macromolecules, including proteins, lipids, and nucleic acids (Gabriele et al., 2017). Therefore, the cellular enzymes accountable for the activity of antioxidants, specifically SOD and CAT, significantly neutralize ROS, thereby reducing oxidative stress. The results of this study suggest a correlation between the reproductive toxicity caused by PB and oxidative stress, as seen by a reduction in the functionality of antioxidant enzymes such as SOD and CAT. This observation indicates the existence of considerable oxidative stress. Our results agree with Venditti et al. (1998), who reported an increase in MDA and a significant decrease in antioxidant levels in rats administered PB.
A study by Mostafa et al. (2012) postulated that natural antioxidants possess a protective mechanism against the harmful effects induced by PB. Another study performed by Rasool et al. (2021) showed a significant enhancement in the oxidative damage induced by ethylene oxide in rats after the administration of CB. The introduction of CB following PB intake led to a notable elevation in the enzymatic activity of SOD and CAT inside the testes, suggesting a positive enhancement in the antioxidant capacity. The results of this study are consistent with prior research that has observed comparable improvements in antioxidant enzyme activity after the introduction of CB or Vit-C to laboratory animals (Kim et al., 2008). The protective effect against lipid peroxidation seen after the administration of CB and/or Vit-C following PB gavage intake may be attributed to their capacity to scavenge free radicals. The findings of this study are consistent with prior research that has shown the efficacy of CB and Vit-C in reducing oxidative lipid damage induced by hazardous agents in animal models (Hermans et al., 2020; Faheem et al., 2020; Dosedˇel et al., 2022). According to the studies carried out by Hussain et al. (2017) and Kurpik et al. (2021), the utilization of CB-extract by the administration exhibited a favourable impact on the changes detected in blood oxidative stress indicators in rats subjected to the CCl4 model. Previous studies have provided evidence of the beneficial effects of CB extract on circumstances generated by oxidative stress in rats (Faheem et al., 2020; Kurpik et al., 2021).
The study’s results revealed that administering PB in rats significantly decreased sperm count, motility, and vitality compared to the control group. The influence of oxidative stress on testicular function and the ability of antioxidants to mitigate this damage has been established by Asadi et al. (2017). The simultaneous administration of CB and Vit-C significantly improved sperm count, motility, and vitality compared to rats treated alone with PB. According to the research conducted by Rasool et al. (2021), their findings indicate that CB-extract may contribute to promoting spermatogenesis through its ability to maintain the antioxidant in a biologically active state efficiently. An observed deficiency in Vit-C has been found to induce oxidative stress in the testes, hence affecting the physiological mechanisms involved in testosterone synthesis and spermatogenesis. The hydro ascorbate reductase enzyme, which relies on GSH for its activity, plays a crucial role in maintaining Vit-C in its reduced form. This enzyme is particularly abundant in the testis. Vit-C is a widely recognized antioxidant occurring naturally within the testicular tissue. The fundamental purpose of this mechanism is to protect the tissues of the testes by reducing the harmful effects of oxidative damage, as stated by Sanghishetti et al. (2014).
Vit-C reduction is facilitated by the GSH-dependent dehydro ascorbate reductase enzyme, which is abundantly present in the testis. Prior research has indicated that Vit-C may enhance sperm motility and the quality of semen and fertility in rats (Rekha et al., 2009; Sanghishetti et al., 2014). Furthermore, including Vit-C supplements in the diets of rats has been extensively practised, enhancing their production and reproductive capabilities, leading to a notable increase in the dosage supplied. Sanghishetti et al. (2014) recorded a study that found that including Vit-C in an individual’s dietary intake has shown a potential to augment sperm quantity and concentration.
The present study has established that PB exposure can trigger testicular harm. The CB and/or Vit-C treatment ameliorated testicular dysfunctions and decreased histological abnormalities induced by PB exposure. The study’s results revealed that rats subjected to PB exposure experienced degeneration of testicular tissue, disruption of cellular structure, and impairment of spermatogenic cells. Administration of CB and/or Vit-C has demonstrated a mitigating impact on the testicular histological changes induced by PB therapy. The observed result can be ascribed to the possible antioxidant properties of CB and Vit-C, as evidenced by prior research (Hermans et al., 2020; Faheem et al., 2020).
Furthermore, the results revealed that rats administered with PB significantly increased the immunohistochemical expression of caspase-3 in the testicular tissue. The findings of our investigation indicate that the occurrence of testicular toxicity generated by phenobarbital can be attributed to the underlying mechanisms of apoptosis and oxidative stress. A prior investigation examining the impacts of the antiepileptic drug valproic acid also revealed its capacity to initiate programmed cell death, known as apoptosis, in granulosa cells of both human and rat subjects. This result was substantiated by increased caspase-3 activity (Mohamed, 2021). The administration of CB or Vit-C decreased caspase-3 expression in the testis, likely due to their anti-apoptotic actions. In a prior investigation conducted by Gholam (2016), it was observed that the co-administration of Vit-C and Acyclovir reduced the prevalence of caspase-3 expressions.
Moreover, the findings of this study align with the outcomes published by Safaa et al. (2020). Gedik and Collins (2005) propose that oxidative stress plays a significant role in amplifying DNA damage. Skrovankova et al. (2015) propose that using CB may alleviate the deleterious effects of free radicals and apoptosis. DNA is frequently subjected to the detrimental effects of many substances, resulting in its deterioration. Nevertheless, the detrimental effects can be alleviated by incorporating dietary supplements and vitamins that offer ample antioxidants (De Martinis & Bianchi, 2001). Furthermore, it is commonly acknowledged within the scientific community that antioxidants are pivotal in protecting tissues from activated oxygen species’ detrimental impacts, as Rahman et al. (2012) emphasized. This study effectively showcased the capability of CB and Vit-C, both solely or/ and in conjunction, to safeguard the testis from toxicity induced by daily administration of phenobarbital (PB) over one month.
While the provided data does not explicitly mention Vit- C is a potent antioxidant that can neutralize reactive oxygen species (ROS) and protect cells from oxidative stress. The combination of folate and Vit- C synergistically enhances antioxidant defence mechanisms and reduces the oxidative stress induced by phenobarbital, thereby mitigating its reproductive toxicities. The strong binding affinities and specific interactions of phenobarbital with proteins like CASP3, GSHB, SOD, and THAS may contribute to its reproductive toxicity by disrupting cellular processes such as apoptosis, redox balance and antioxidant defence mechanisms. Conversely, through their interactions with these proteins and their inherent antioxidant properties, folate and Vit- C may counteract the adverse effects of phenobarbital and provide protective effects against reproductive toxicities. However, further experimental evidence and mechanistic studies would be required to establish a definitive link between these interactions and the observed protective effects of the co-treatment
Acknowledgements
The authors thank the members of the institutional animal care committee of the Faculty of Science at Tanta University and the members of the Zoology Department, Faculty of Science, Cairo University, for their helpful suggestions during the preparation of this study.
Author Contributions
All authors are equally contributed to this study.
Disclosure Statement
The authors declare no conflicts of interest.
References
| 1 | Abd-Elhafeez, H.H., Soliman, S.A., Attaai, A.H., Abdel-Hakeem, S.S., El-Sayed, A.M., and Abou-Elhamd, A.S. (2021): Endocrine, stemness, proliferative, and proteolytic properties of alarm cells in ruby-red-fn shark (Rainbow Shark), Epalzeorhynchos frenatum (Teleostei: Cyprinidae). Microsc Microanal. 27(5): 1251-1264.
https://doi.org/10.1017/S1431927621012265 |
| 2 | Aebi, H. (1984): Catalase in vitro Methods Enzymol. 204: 234-254.
|
| 3 | Ali, K., Abdelrazik, M., Alghaithy, A., Diab, A., El-Beshbishy, H., Baghdadi, H. (2014): Antimutagenic and Anticancer Activity of Al Madinah Alhasawy Mint (Mentha longifolia) Leaves Extract. Pakistan journal of biological sciences: PJBS. 17: 1231-126.
https://doi.org/10.3923/pjbs.2014.1231.1236 |
| 4 | Asadi, N., Bahmani, M., Kheradmand, A., and Rafieian-Kopaei, M. (2017): The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. Journal of Clinical and Diagnostic Research. 11(5): 1-5.
https://doi.org/10.7860/JCDR/2017/23927.9886 |
| 5 | Bancroft, J.D., and Gamble, M. (2002). Theory and practice of histological techniques.5th. Ed. Edinburgh. Churchill Livingstone Pub. 172-5, pp 593-620.
|
| 6 | Beutler, E., Duron, O., and Kelly, B.M. (1963): Improved method for the determination of blood glutathione. J Lab Clin Med. 61: 882-890.
|
| 7 | BIOVIA Discovery Studio (2020). Dassault Systèmes BIOVIA, San Diego, CA, USA.
|
| 8 | Blumberg, J.B., Camesano, T.A., Cassidy, A., Kris-Etherton, P., Howell, A., Manach, C. (2013): Cranberries and their bioactive constituents in human health. Advances innutrition. 4: 618-632.
https://doi.org/10.3945/an.113.004473 |
| 9 | Boušová, I., Bártíková, H., Matoušková, P., Lnˇeniˇcková, K., Zappe, L., Valentová, K., Szotáková, B., Martin, J., and Skálová, L. (2015): Cranberry extract-enriched diets increase NAD(P)H:quinone oxidoreductase and catalase activities in obese but not in non-obese mice.Nutr. Res. 35: 901-909.
https://doi.org/10.1016/j.nutres.2015.08.002 |
| 10 | Brodie, M.J., and Kwan, P. (2012): Current position of phenobarbital in epilepsy and its future. Epilepsia. 53(8): 40-6.
https://doi.org/10.1111/epi.12027 |
| 11 | Cesonienė, L., and Daubaras, R. (2015): Phytochemical Composition of the Large Cranberry (Vaccinium macrocarpon) and the Small Cranberry (Vaccinium oxycoccos). 10.1016/B978-0-12-408117-8.00008-8.
https://doi.org/10.1016/B978-0-12-408117-8.00008-8 |
| 12 | De Martinis BS, and Bianchi MD. (2001): Effect of vitamin C supplementation against cisplatin - induced toxicity and oxidative DNA damage in rats. Pharmacol Res. 44(4):317 - 3 20.
https://doi.org/10.1006/phrs.2001.0860 |
| 13 | Dosed, M., Jirkovský, E., Macáková, K., Krˇcmová, L.K., Javorská, L., Pourová, J., Mercolini, L., Remião, F., Nováková, L., and Mladˇenka, P. (2021): Vit-C sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients. 13: 615.
https://doi.org/10.3390/nu13020615 |
| 14 | Esterbauer, H., and Cheeseman, K.H. (1990): Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 186:407-421.
https://doi.org/10.1016/0076-6879(90)86134-H |
| 15 | Faheem, S.A., Saeed, N.M., El-Naga, R.N., Ayoub, I.M., and Azab, S.S. (2020): Hepatoprotective effect of cranberry nutraceutical extract in non-alcoholic fatty liver model in rats: impact on insulin resistance and Nrf-2 expression. Front. Pharmacol. 11: 218.
https://doi.org/10.3389/fphar.2020.00218 |
| 16 | Falsaperla, R., Mauceri, L., Pavone, P., Barbagallo, M., Vitaliti G, and Ruggieri, M. (2019): Short-term neurodevelopmental outcome in term neonates treated with phenobarbital versus levetiracetam: a single-center experience. Behav Neurol. 2019: 3683548.
https://doi.org/10.1155/2019/3683548 |
| 17 | Gabriele, P., Natasha, I., Mariapaola, C., Giovanni, P., Federica, M., Vincenzo, A., Francesco, S., Domenica, A., and Alessandra, B. (2017): Oxidative stress: harms and benefits for human health. Oxidative Medicine and Cellular Longevity 2017: Article ID 8416763 1-13.
https://doi.org/10.1155/2017/8416763 |
| 18 | Gedik, C.M., and Collinsm C.(2005):Establishing the background level of base oxidation in human lymphocyte DNA: results of an interlaboratory validation study.FASEB J.19:82-84.
https://doi.org/10.1096/fj.04-1767fje |
| 19 | Hanwell, M. D., Curtis, D. E., Lonie, D. C., Vandermeersch, T., Zurek, E., & Hutchison, G. R. (2012). Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. Journal of Cheminformatics, 4(1), 1-17.
https://doi.org/10.1186/1758-2946-4-17 |
| 20 | Hermans, M., Charalambous, M., Pakozdy, A. (2022): Evaluation of the effect of phenobarbital administration on the biochemistry profile, with a focus on serum liver values, in epileptic cats. Journal of Feline Medicine and Surgery. 24(6): 530-538.
https://doi.org/10.1177/1098612X211037431 |
| 21 | Hussain, F., Malik, A., Ayyaz, U., Shafique, H., Rana, Z., Hussain, Z. (2017): Efficient hepatoprotective activity of cranberry extract against CCl4.Asian Pac. J. Trop. Med. 10: 1054-1058.
https://doi.org/10.1016/j.apjtm.2017.10.008 |
| 22 | Kim, M.J., Jung, H.N., Nam, K.N., and Kwak, H.K. (2008): Effects of cranberry powder on serum lipid profiles and biomarkers of oxidative stress in rats fed an atherogenic diet. Nutr Res. 2(3): 158-164.
https://doi.org/10.4162/nrp.2008.2.3.158 |
| 23 | Kurpik, M., Zalewski, P., Kujawska, M., Ewertowska, M., Ignatowicz, E., Cielecka-Piontek, J., Jodynis-Liebert, J. (2021): Can CranberryJuice Protect againstRotenone-Induced Toxicity in Rats?Nutrients. 13: 1050.
https://doi.org/10.3390/nu13041050 |
| 24 | Lapshina E.A., Zamaraeva M., Cheshchevik V.T., Olchowik-Grabarek, E. Sekowski S., and Zukowska, I. (2015): Cranberry flavonoids prevent toxic rat liver mitochondrial damage in vivo and scavenge free radicals in vitro Cell Biochem Funct. 33: 202-210.
https://doi.org/10.1002/cbf.3104 |
| 25 | Lewis, C.B., and Adams, N. (2022): Phenobarbital. In: StatPearls. Treasure Island (FL): StatPearls Publishing; Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532277.
|
| 26 | Marcus D Hanwell, Donald E Curtis, David C Lonie, Tim Vandermeersch, Eva Zurek and Geoffrey R Hutchison; "Avogadro: An advanced semantic chemical editor, visualization, and analysis platform" Journal of Cheminformatics 2012, 4:17.
https://doi.org/10.1186/1758-2946-4-17 |
| 27 | MohamedI., Mustafa, M.,Mohamad, S.,Yahia, R.,ABdel -aziz, M.,Abuo -Rahma, G. Hayallah, A. (2021): Novel Mannich Bases of Ciprofloxacin with Improved Physicochemical Properties, Antibacterial, Anticancer Activities and Caspase-3 Mediated Apoptosis. Bioorganic Chemistry. 107 104629 10.1016/j.bioorg.2 021.104629.
https://doi.org/10.1016/j.bioorg.2021.104629 |
| 28 | Morakinyo, A. O., Iranloye, B. O., & Adegoke, O. A. (2009). Antireproductive effect of calcium channel blockers on male rats. Reproductive Medicine and Biology, 8, 97-102 https://doi.org/10.1007/s1252 2-009-0018-9
https://doi.org/10.1007/s12522-009-0018-9 |
| 29 | Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., & Olson, A. J. (2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Journal of Computational Chemistry, 30(16), 2785-2791.
https://doi.org/10.1002/jcc.21256 |
| 30 | Mostafa, R.M., Moustafa, Y.M., and Mirghani, Z. (2012): Thymoquinone alone or in combination with phenobarbital reduces the seizure score and the oxidative burden in pentylenetetrazole-kindled ratsOxid Antioxid Med Sci. 1(3): 185-192.
https://doi.org/10.5455/oams.031012.or.021 |
| 31 | Nemzer, B. V., Al-Taher, F., Yashin, A., Revelsky, I., & Yashin, Y. (2022). Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health: Overview. Molecules (Basel, Switzerland), 27(5), 1503.
https://doi.org/10.3390/molecules27051503 |
| 32 | Newman, D.J., and Cragg G.M. (2020): Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019 J. Nat. Prod. 83: 770-803.
https://doi.org/10.1021/acs.jnatprod.9b01285 |
| 33 | Nishikimi, M., Rao, N.A., and Yagi, K. (1972): The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochemical and Biophysical Research Communications. 46: 849-85.
https://doi.org/10.1016/S0006-291X(72)80218-3 |
| 34 | Padayatty, S.J., and Levine, M. (2016): Vit-C: The known and the unknown and Goldilocks.Oral. Dis. 22: 463-493.
https://doi.org/10.1111/odi.12446 |
| 35 | Rahim, F., Azizimalamiri, R., Sayyah, M., and Malayeri, A. (2021): Experimental Therapeutic Strategies in Epilepsies Using Anti-Seizure Medications. Experim Pharmacol. 13: 265-290.
https://doi.org/10.2147/JEP.S267029 |
| 36 | Rahman, T., Hosen, I., Islam, and Towhidul S.H. (2012): Oxidative stress and human health. Advances in Biosci ence and Biotechnology. 03 997 - 1019.
https://doi.org/10.4236/abb.2012.327123 |
| 37 | Rasool, M., Malik, A., Ashraf, M.B., Mubbin, R., Ayyaz, Y., Waquar, S., Asif, M., Umar, M., Hua, G.S., Iqbal, Z., Alamh, H., and Achakzai, N.M. (2021): Phytochemical analysis and protective effects of Vaccinium macrocarpon (cranberry) in rats (Rattus norvegicus) following ethylene oxide-induced oxidative insult. Bioengineered. 12: 4593-4604.
https://doi.org/10.1080/21655979.2021.1955528 |
| 38 | Rekha, D.K., Nayanatara, A.K., Ramaswamy, R.P., Ramesh, B., and Venkappa, S.M. (2009): Infertility in male wistar rats induced by cadmium chloride: role of ascorbid acid. Journal of Chinese clinical medicine. 4(11): 616-621.
|
| 39 | Robb, G.W., Amann, R.P., and Killian, G.J. (1978): Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil. 54(1):103-107.
https://doi.org/10.1530/jrf.0.0540103 |
| 40 | Safaa A., Gewaily, M., Abo - Al - Ela, H., Almeer, R., Soliman, A., Elkomy, A., Dawood, M. (2021): Vit-C rescues inflammation, immunosuppression, and histopathological alterations induced by chlorpyrifos in Nile tilap ia. Environmental Science and Pollution Research. 28 1 - 14 10.1007/s11356 - 021 - 12711 - 5.
|
| 41 | Sanghishetti, V., Ghongane, B., and Nayak, B. (2014): Effect of Vit-C on Male Fertility in Rats Subjected to Forced Swimming Stress. Journal of clinical and diagnostic research. 8: 5-8.
|
| 42 | Sauter, E.R. (2020): Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev Clin Pharmacol. 13: 265-285.
https://doi.org/10.1080/17512433.2020.1738218 |
| 43 | Singh, G., Passari, A., Leo, V., Mishra, V., Subbarayan, S., Kumar, B., Singh, B., Kumar, S., Lalhlenmawia, H., Gupta, V. and Nachimuthu, S.K. (2016): Evaluation of phenolic content variability, antioxidant, antimicrobial and cytotoxic potential of selected traditional medicinal plants from India. Frontiers in Plant Science. 7 407 10.3389/fpls.2016.00407.
https://doi.org/10.3389/fpls.2016.00407 |
| 44 | Skrovankova S, Sumczynski D, Mlcek J, Jurikova T, Sochor J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int J Mol Sci. 2015 Oct 16;16(10):24673-706.
https://doi.org/10.3390/ijms161024673 |
| 45 | Slott, V.L., Suarez, J.D., and Perreault, S.D. (1991): Rat sperm motility analysis: methodologic consideq rations. Reprod Toxicol.5(5): 449-458.
https://doi.org/10.1016/0890-6238(91)90009-5 |
| 46 | Stobnicka, A., and Gniewosz, M. (2018): Antimicrobial protection of minced pork meat with the use of Swamp Cranberry (Vaccinium oxycoccos L.) fruit and pomace extracts. Food Sci Tech. 55: 62-71.
https://doi.org/10.1007/s13197-017-2770-x |
| 47 | Trott, O., & Olson, A. J. (2010). AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), 455-461.
https://doi.org/10.1002/jcc.21334 |
| 48 | Venditti, P., Daniele, C.M., De Leo, T., and0020Meo, S.D. (1998): Effect of phenobarbital treatment on characteristics determining susceptibility to oxidants of homogenates, mitochondria and microsomes from rat liver. Cell Physiol Biochem. 18(6): 328-338.
https://doi.org/10.1159/000016294 |
| 49 | Vijayprasad, S. (2014): Effect of Vit-C on male fertility in rats subjected to forced swimming stress. Clinical and diagnostic research: JCDR. 8: 5-8.
https://doi.org/10.7860/JCDR/2014/8432.4622 |
| 50 | World Health Organization. (WHO) (2013): Traditional medicine strategy 2014-2023.
|
| 51 | Xueni, S., Yintao, Z., Ying, Z., Xichen, L., Lili, Y., Ting, P., Ting, J., Han, X., Zimao, L., Wenqi, Q., Jianxin, W., Zhaorong, L., Feng, Z., and Xinbing, S. (2022): NPCDR: natural product-based drug combination and its disease-specific molecular regulation, Nucleic Acids Research. 50: 1324-1333.
https://doi.org/10.1093/nar/gkab913 |
| 52 | Yang Liu, Xiaocong Yang, Jianhong Gan, Shuang Chen, Zhi-Xiong Xiao, Yang Cao, CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting, Nucleic Acids Research, Volume 50, Issue W1, 5 July 2022, Pages W159-W164.
https://doi.org/10.1093/nar/gkac394 |