Accurate and Efficient Algorithm for Detection of Alzheimer Disability Based on Deep Learning
bJoint Institute for Nuclear Research, 141980 Dubna, Russia
cAcademy of Scientific Research & Technology (ASRT), Egypt
dEngineering Department, NRC, Egyptian Atomic Energy Authority, Egypt,
Keywords
Abstract
Background/Aims:
Alzheimer’s Disease (AD) is a progressive neurodegenerative disorder that severely affects cognitive functions and memory. Early detection is crucial for timely intervention and improved patient outcomes. However, traditional diagnostic tools, such as MRI and PET scans, are costly and less accessible. This study aims to develop an automated, cost-effective digital diagnostic approach using deep learning (DL) and computer-aided detection (CAD) methods for early AD identification and classification.Methods:
The proposed framework utilizes pretrained convolutional neural networks (CNNs) for feature extraction, integrated with two classifiers: multi-class support vector machine (MSVM) and artificial neural network (ANN). A dataset categorized into four groups—non-demented, very mild demented, mild demented, and moderate demented—was employed for evaluation. To optimize the classification process, a texture-based algorithm was applied for feature reduction, enhancing computational efficiency and reducing processing time.Results:
The system demonstrated high statistical performance, achieving an accuracy of 91%, precision of 95%, and recall of 90%. Among the initial set of twenty-two texture features, seven were identified as particularly effective in differentiating normal cases from mild AD stages, significantly streamlining the classification process. These results validate the robustness and efficacy of the proposed DL-based CAD system.Conclusion:
This study presents a reliable and affordable solution for early AD detection and diagnosis. The proposed system outperforms existing state-of-the-art models and offers a valuable tool for timely treatment planning. Future research should explore its application to larger, more diverse datasets and investigate integration with other imaging modalities, such as MRI, to further enhance diagnostic precision.Introduction
Both health and life expectations are increased due to the development of medical science and healthcare [1]. However, the AD is the main form of dementia [2] because of the neurodegeneration [3]. Also, it appears in the form of memory loss and cognitive issues [1]. Much more is known about AD and its effects on people and communities now than twenty years ago [4-5]. There are substantial healthcare issues associated with AD, a neurological ailment that impacts millions of individuals globally [4]. Cognitive deterioration, memory loss, behavioral and behavioral abnormalities, and other symptoms are hallmarks of this condition [5]. To intervene early and effectively manage AD, a diagnosis is essential. Brain abnormalities associated with AD have been detected using a variety of imaging techniques, such as PET and MRI [6-9]. The structural and functional alterations in the brain can be better understood with the help of these imaging modalities, which in turn helps with accurate diagnosis and tracking the progression of disease [6-7]. Additionally, recent developments in CAD systems have demonstrated the possibility of automatically analyzing images of AD. It will greatly benefit physicians in their evaluations [10-11]. The use of complex algorithms in these CAD systems allow for the improved detection [12] of AD biomarkers and patterns, leading to more efficient and accurate diagnoses [10-11].
Accurately detecting mild in AD images automatically through CAD is a significant task. To describe the pathogenesis of AD, several methods have been employed. MRI and PET are two imaging modalities that have successfully depicted the structural and functional alterations linked to AD [6-7, 13]. On the other hand, conventional roentgenographic methods have sparked worries about radiation exposure levels [14]. The existence of many types of noise, such as Gaussian, Poisson, Salt and Pepper, Speckle, and passion noise, makes it difficult to detect AD from images [15-16]. Because of this delay in diagnosis, patients may go untreated for critical mild, increasing their risk of additional breaks [15]. Because of this, effective algorithms for spotting these cracks in AD pictures are urgently required. Using the original image’s properties, like power spectral density (PDS) and higher-order statistics (HOS), is one way to detect mild images of AD [17]. Using cepstral coefficients, this method achieves accurate detection [17-18]. One benefit of using spectral subtraction is that it can reduce the amount of extra distortion in the final product [17, 19].
This article aims to tackle the monumental task of automatically recognizing mild in images of AD utilizing CAD systems. The purpose of this article is to suggest a practical technique for the precise detection of mild in images of AD. Imaging for AD cannot be done using the standard radiographs that are used to evaluate musculoskeletal diseases. Therefore, the article’s main focus is on creating a new method for detecting mild in AD images using CAD and modern image processing techniques [9]. Using PDS and HOS, which are features taken from the original image, the authors hope to circumvent the shortcomings of conventional imaging methods. To detect mild in images of AD, they suggest incorporating cepstral coefficients into the algorithm. Presenting a dependable and precise method for automatically finding diseases in images of AD is the ultimate purpose of the paper. The suggested approach may improve diagnosis and help create better tools for controlling AD if it can accomplish this goal.
DL, a subset of machine learning, utilizes ANNs to process and analyze complex data. ANNs are inspired by the human brain’s structure, consisting of interconnected layers of nodes (neurons) that process information in a hierarchical manner. This architecture enables ANNs to effectively recognize patterns in structured data, making them invaluable in medical diagnostic applications. CNNs, a specialized form of ANNs, are designed specifically for image analysis. CNNs employ convolutional layers to automatically detect spatial hierarchies within images—ranging from basic elements like edges and textures to more intricate patterns—by learning through filters. This hierarchical feature extraction from raw pixel data makes CNNs particularly effective for medical image classification, including AD diagnosis. In this study, pretrained CNNs such as AlexNet, ResNet-18, and VGG-19 were utilized to extract features from AD imaging datasets. These features were then classified using MSVM and ANNs to assess the severity of AD. This approach highlights the capability of pretrained CNNs to support accurate and efficient AD diagnosis [20-21].
This article is concerned with the affordable automatic detection and classification of AD using valuable dataset images instead of MRI/PET images. Scarce models are built to detect this widespread disease using AD images. This innovative technology depends on the DL technology. The features are extracted using the pretrained CNN using AlexNet, ResNet-18, and VGG-19 during training and testing purposes of the AD based on underlined images. The MSVM and ANN are applied as classifiers [22-23].
The use of underlined dataset images for diagnoses of AD provides too much affordable results in comparison with neuroimaging technology. It overcomes the access limits originated by the advanced imaging techniques. The features of these images are extracted using CNN technique. The classification accuracy is evaluated using two different classifiers. These classifiers ANN and MSVM which are linked to the CNN network [24]. Additionally, the results of the proposed algorithms are verified with literature work. Finally, the suggested solution for detection and classification of AD using dataset images decreases both cost and the computational complexity. This article provides the probability of implementation in limited resource environments. Accordingly, it allows the AD diagnoses further reachable.
This paper is organized as follows: Section 2 presents the proposed identification approach for detecting AD images using texture feature. In Section 3, the principle of utilizing DL for detection of AD is briefly discussed. Furthermore, Section 5 presents the obtained results and provides a discussion. Finally, Section 6 concludes this work.
Materials and Methods
AD identification based on textural features with SVM
A program code is implemented to compare the extracted features for normal and Alzheimer images, as shown in Fig. 1. Features are extracted from several multiple AD images by texture features. The average of these features are computed and compared for normal and Alzheimer images. The SVM is employed as a classifier [12]. The texture features are well described in [12, 25].
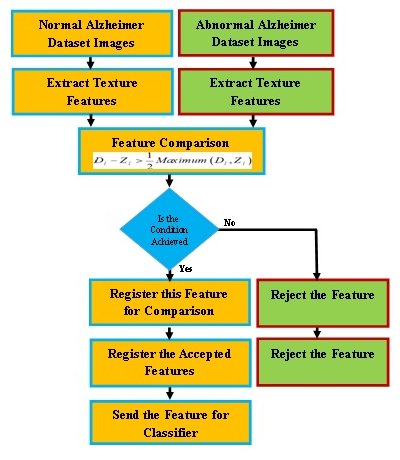
Fig. 1: Early detection of AD using textures feature extractors.
Proposed detection algorithms for Alzheimer’s disability Alzheimer’s detection using DL with neural network.
This research proposes an algorithm to identify mild in AD demented images. The core method leverages a CNN alongside a technique known as data augmentation to enhance detection accuracy as indicated in Fig. 2. Data preparation involves establishing a storage system for the images. Each image is assigned a class label, and the proportion of each class within the dataset is defined. A CNN is then built to extract crucial features from the images. The specific architecture of the CNN is established. To train the model, the images are used to train an ANN. During this process, the ANN’s specifications are fine-tuned. Finally, the effectiveness of the initial model (baseline classifier) is evaluated using a separate validation set to assess its accuracy in Alzheimer’s detection.
The baseline model is enhanced by incorporating data augmentation. This method involves creating a new dataset of modified images through random transformations like horizontal flips, slight shifts, and cropping. The ANN is applied for training of the augmented dataset. The resulting accuracy is evaluated. Next, transfer learning is employed without data augmentation. A pre-trained convolutional neural network called AlexNet is utilized [25]. One of the main strengths of AlexNet is its capability of classifying large datasets of mild AD images. This pre-trained network offers a strong foundation due to the valuable features it has already learned from a vast amount of data. AlexNet typically accepts images with a specific size (227x227 pixels) and can classify objects within numerous categories. While AlexNet is known for its speed and ability to handle smaller datasets, its final layers are replaced to adapt to the specific features within the current dataset. This modified network is then trained using an ANN with adjusted training parameters, and its accuracy is assessed. Finally, the approach combines transfer learning with data augmentation. Here, the dataset images are first augmented to the required size (227x227 pixels) and then fed into the pre-trained AlexNet model.
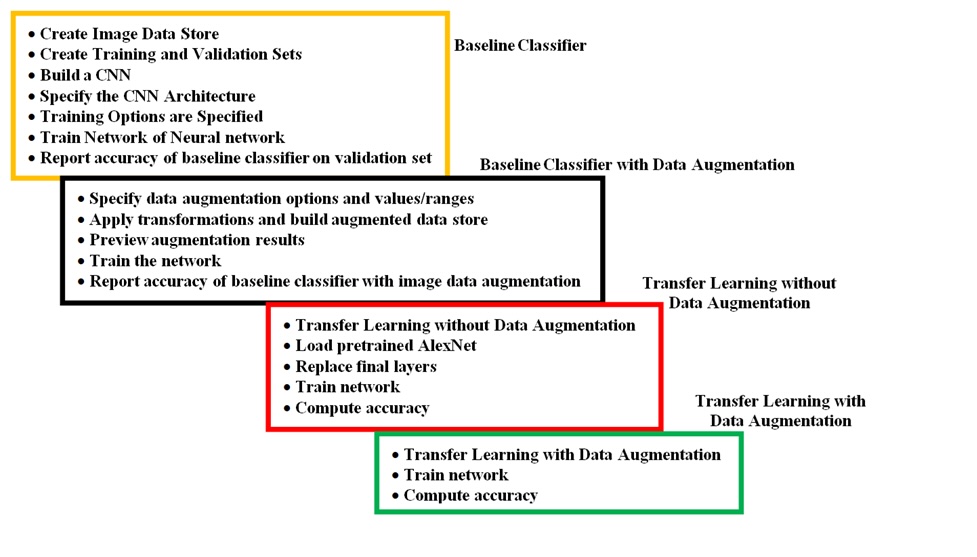
Fig. 2: Alzheimer detection using CNN and data augmentation.
Alzheimer detection using DL with multiclass SVM classifier
This approach capitalizes on the strengths of pre-trained CNNs [26]. These powerful networks have the capability to automatically extract valuable features from data. The extracted features from the pertained CNN are directed to appropriate classifier for training purposes. The inherent capabilities of pre-trained networks allow for a simpler and faster feature extraction process compared to manual feature engineering [26].
A SVM serves as the chosen classifier. It is a type of machine learning algorithms that excels with their proficiency in identifying patterns within datasets [27]. They achieve this by establishing a hyperplane that effectively separates positive and negative examples within the data. This hyperplane is strategically positioned to maximize the margin between the two classes [27].
SVMs address the task of classification by employing an optimal hyperplane within a feature space to separate two distinct sets of data [28]. This hyperplane is strategically positioned to maximize the margin, which is the distance between the closest data points (support vectors) from each class to the hyperplane [28]. While SVMs are primarily designed for binary classification, they can be adapted to handle multi-class problems through various techniques. In its core functionality, an SVM seeks a linear separating function (φ(x) = µΔ + Φ) that aligns with the hyperplane equation (µΔ + Φ = 0) [27]. Here, µ represents the slope and Φ signifies the intercept of the hyperplane. Real-world data often exhibits non-linear relationships. To address this challenge, SVMs commonly employ kernel functions. These functions essentially map the non-linear data from the original space into a higher-dimensional space where linear separation becomes possible [27]. Common kernel functions include Radial Basis Function (RBF), Gaussian, linear, and sigmoid functions [27]. In scenarios where data is inherently non-separable, even with the aid of kernel functions, SVMs introduce a cost function. This function incorporates a penalty term for misclassified data points, aiming to minimize the overall classification error [29-30].
$${ \text{Minimize:} ||Q||^2 + K \sum_{n=1}^{m} S_i \hspace{50px} (1)}$$
Subject to the restrictions [30]
$${ y_i (\omega . B_i + \Phi) \geq 1-S_i \hspace{50px} (2)}$$
The effectiveness of the SVM classifier hinges on a cost function that incorporates a regularization parameter (S) for each data point (Bi) [29-30]. This parameter plays a crucial role in handling potential misclassifications.
- Positive λ i (0 ≤ λ i < 1): When a data point falls within the correct classification range, the corresponding λi value is positive and less than 1. This indicates a correctly classified point and contributes minimally to the cost function.
- λ i greater than 1: If a data point is misclassified, the λi value becomes greater than 1. This penalizes the model for the error and encourages the algorithm to adjust its decision boundary to minimize such misclassifications.
This research proposes an algorithm for identifying mild from demented AD images as demonstrated in Fig. 4. The method employs pre-trained CNNs including AlexNet, ResNet-18, and VGG-19. The loaded dataset includes both normal and demented AD images. The demented images comprise three different categories that are mild, moderate and very mild AD images. To ensure effective training, the sizes of the two image categories (normal and demented) within the dataset might require balancing. This can be achieved by adjusting the number of images in each category. The pre-trained CNNs function as feature extractors. They analyze the images and learn distinctive patterns that differentiate between normal and demented AD images. Subsequently, a SVM classifier is trained using the features extracted by the CNNs. This classifier learns to distinguish between the two categories based on the learned features. The effectiveness of the approach is assessed by comparing the performance of the SVM classifier when utilizing different pre-trained CNN models. This variation of the pre-trained CNN network leads to the most accurate results in identifying demented AD images.
This work utilizes a pre-trained CNN architecture known as VGG-19. This powerful network has been trained on a massive dataset encompassing thousands of object categories. VGG-19 boasts a deep structure consisting of 19 convolutional layers, allowing it to capture complex features from the input images. By leveraging a pre-trained VGG-19 model, this approach benefits from the extensive knowledge the network has already acquired through prior training on a large dataset. The pre-trained VGG-19 acts as a feature extractor in this application. It analyzes the AD dataset images and extracts informative features that differentiate between normal and demented images. A hidden layer within the model is then trained using a training set from the available database. The model’s performance is evaluated using a loss function, which measures the discrepancy between the model’s predictions and the actual labels. Minimizing this loss function is crucial for model optimization. This work employs the stochastic gradient descent (SGD) optimizer for model optimization. SGD is a widely used algorithm known for its efficiency and ability to handle noisy or sparse gradients. While SGD is a valuable choice, other optimizers like Adam also offer advantages. Adam is known for its computational efficiency, low memory requirements, and effectiveness in dealing with noisy data [31].
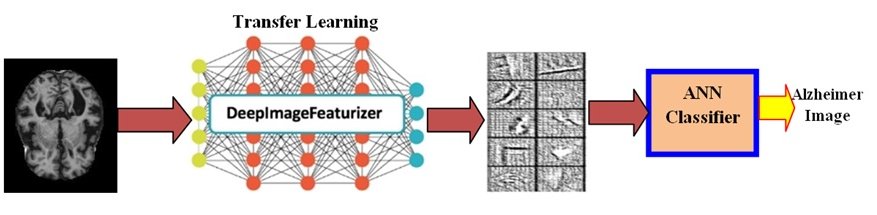
Fig. 3: CNN of a pretrained network.

Fig. 4: AD detection algorithm from normal and demented images using DL.
Results
The accuracy of the suggested algorithms heavily relies on established pretrained DL networks. To investigate these algorithms, MATLAB 2020b environment, specifically the DL toolbox, was utilized. These algorithms can be executed on a laptop computer equipped with an Intel Core I5-4300M@2.5GHz CPU, 8GB of RAM, and a 64-bit operating system. All experiments were conducted using MATLAB 2020b on a Windows 10 environment.
The experiments were implemented using four distinct datasets comprising mild demented, moderate demented, non-demented, and very mild demented images. These dataset images were sourced from various websites [32]. The training AD datasets represent 70% from all input datasets. This amount is enough for learning patterns from AD datasets. While, the remaining datasets (30%) are concerned for both test (15%) and validation (15%) sets. The test set judges on the capability of the considered model to know and identify unknown datasets image. Finally, the validation it helps to alter and tune the hyperparameters and prohibit the overfitting/underfitting issues.
This study employed a SVM classifier to distinguish between normal and mild AD images. A polynomial kernel function was utilized within the SVM framework. A set of over 22 features was extracted from both normal and mild AD images. The parameters of the SVM classifier were optimized to achieve the lowest generalization error, leading to the best possible performance. Through a program code specifically designed for comparison, the effectiveness of each extracted feature in differentiating between normal and mild AD was evaluated. The analysis revealed that only 7 features out of the initial 22 were obviously effective in differentiating between normal and mild AD images as declared in Table 1. The remaining 15 features yielded similar results and were deemed insufficient for reliable classification, as illustrated in Table 1. Utilizing a smaller set of relevant features can lead to improved classification efficiency due to reduced computational demands.
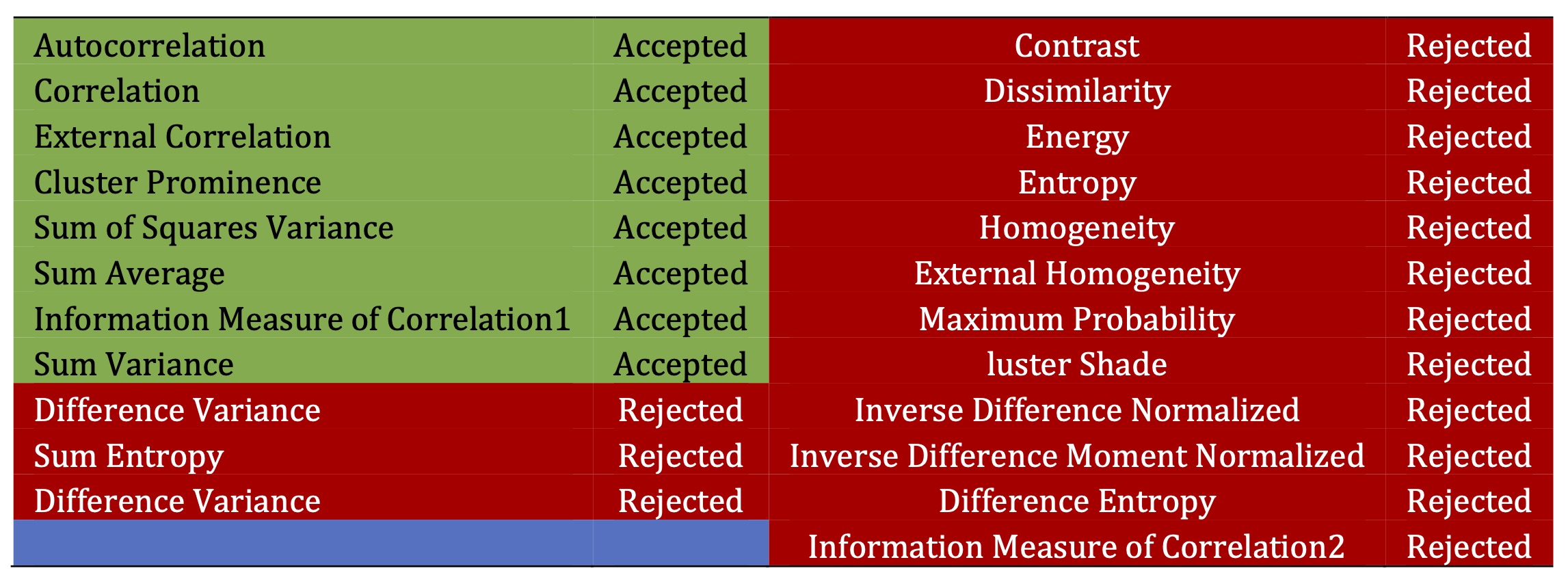
Table 1: Features reduction for normal and mild Alzheimer images
In this context, the performance of pretrained networks such as AlexNet, ResNet18, and VGG19 is evaluated and compared using the dataset images under consideration. Furthermore, these networks are utilized in conjunction with both ANN and MSVM classifiers, which are subsequently tested on the aforementioned dataset images. A comprehensive comparison is conducted by applying these classifiers independently on each pretrained network. The output layer utilizes the learned model within the hidden layer to make predictions. The accuracy (ξ) serves as the primary evaluation criterion for the network models being considered, as stipulated by [31].
$${E = \left( 1 - \left( \frac{1}{y} \sum_{u=1}^{y} \frac{O_u - H_u}{H_u} \right) \right) \times 100 \% \hspace{50px} (3)}$$
In the given context, the variables Hu and Ou represent the target output and prediction value, respectively. The estimation of losses, on the other hand, is determined based on a specific methodology or approach [33-34].
$${ R = (1-E) \hspace{50px} (4)}$$
Fig. 5 (a) presents the accuracy of mild detection for AD images, showcasing how it evolves with the number of Epochs. At iteration number 15, an accuracy of 85% is achieved. Notably, the accuracy progressively increases as the number of iterations grows. Fig. 5 (b) illustrates the losses incurred during the training process using the AlexNet pretrained network with ANN. It is observed that the losses due to false detection decrease with each iteration. This algorithm exhibits higher accuracy in detecting original gray images. Moreover, the accuracy achieved using the ResNet18 pretrained network with ANN is demonstrated across all considered database images, as shown in Fig. 6 (a). This accuracy surpasses that of the AlexNet network. The corresponding losses against training epochs during the training process using the ResNet18 pretrained network and ANN are evident in Fig. 6 (b). The utilization of the ResNet18 pretrained network leads to a reduction in mild detection errors, thereby improving the obtained results. Furthermore, the accuracy is enhanced by incorporating the MSVM classifier.
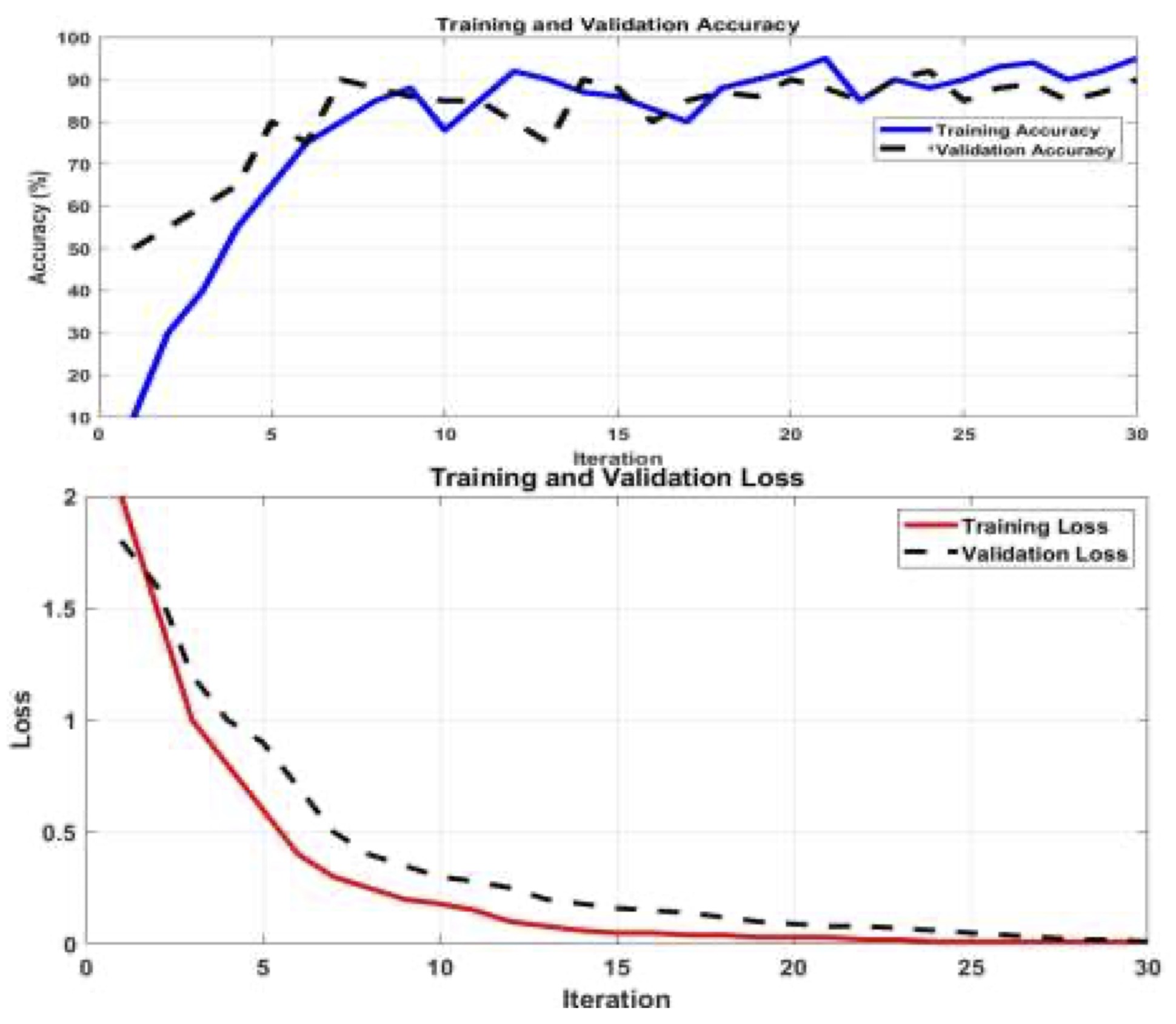
Fig. 5: a) Accuracy against training epochs and b) Losses against the training epochs for AlexNet pretrained network with ANN.
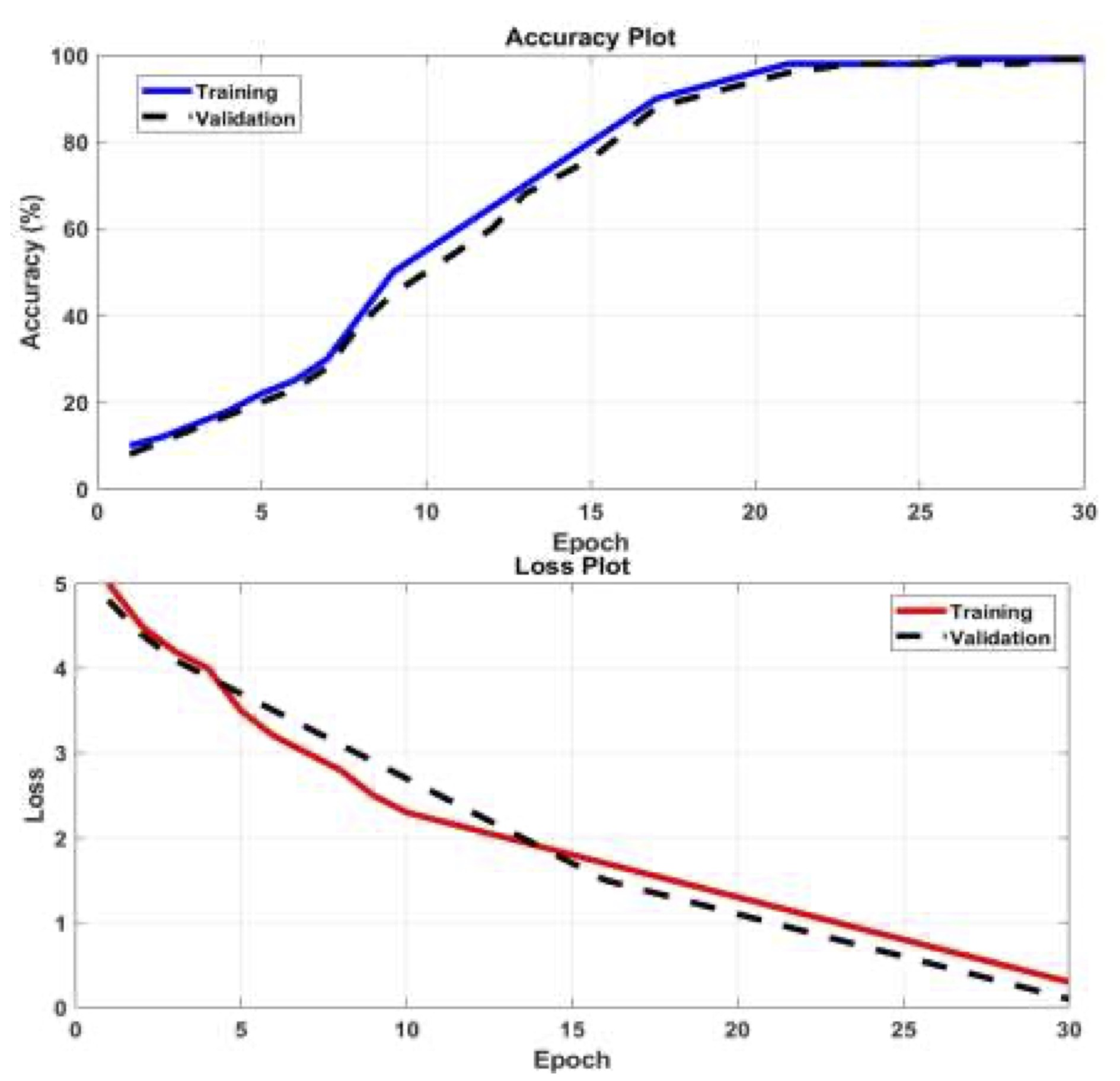
Fig. 6: (a) The accuracy versus training epochs and (b) Loss against training epochs for detecting mild by pre-trained ResNet18 network with ANN.
An extensive comparison between the suggested algorithms is conducted in terms of several metrics such as precision, accuracy, recall, F-score, and specificity. These metrics serve as key indicators of the algorithms’ performance. The classification accuracy, which refers to the total number of correct predictions in relation to the overall number of predictions within the dataset, serves as a fundamental metric for assessing the performance of a specific model. While it is effective for balanced datasets, it may not be reliable for unbalanced datasets. The concept of classification accuracy is defined by [33, 35].
$${ACC = \frac{R_p + R_N}{R_p + L_p + L_N + R_N} \hspace{50px} (5) }$$
In the context of classification evaluation, the terms RP (true positive), RN (true negative), LP (false positive), and LN (false negative) are used. While accuracy is a common metric, it fails to consider the complexities associated with class imbalances. Therefore, precision, recall metrics, and F-score serve as alternative metrics to classification accuracy. Precision, in particular, is a valuable measure when the false positive rate is high. It assesses the correctness of positive predictions and quantifies the deviation of the model’s accuracy from the predicted positive scenarios. Precision can be defined as the fraction of actual true positives identified by the model out of all positively classified examples. The formulation for precision is given by [33, 35].
$${ \text{Precision} = \frac{R_p}{R_p + L_p} \hspace{50px} (6)}$$
The precision metric is a key focus in classification evaluation, aiming for a higher value. Precision is directly related to the true positive rate, emphasizing the correctness of positive predictions. Another important classification metric is recall, which highlights the model’s performance when dealing with false negatives. A higher recall value is desirable, as it is proportional to the number of true positives. The Recall is also referred to as sensitivity and represents the fraction of true positives among the total number of positive examples and false negatives. The calculation for recall is described by [33, 35].
$${ \text{Recall} = \frac{R_p}{R_p + L_N} \hspace{50px} (7)}$$
In scenarios involving class imbalances, the F-score serves as a crucial metric that replaces accuracy. It is particularly useful for evaluating model accuracy in datasets with binary classifications, such as positive or negative outcomes. The F-score combines both precision and recall measures to assess the model’s performance. In other words, it represents the harmonic mean of precision and recall. The F-score is commonly employed in the assessment of information retrieval systems. It becomes the preferred choice when achieving a balance between precision and recall, especially when dealing with a large number of actual negatives. The calculation for the F-score is defined by [33, 35].
$${ \text{Fscore} = \frac{2 R_p}{2 R_p + L_p + L_N} \hspace{50px} (8) }$$
Specificity corresponds to the ability of the program to correctly label individuals. This metric focuses on capturing all true negatives. The calculation for specificity is defined as follows [33].
$${ \text{Specificity} = \frac{R_N}{L_p + R_N} \hspace{50px} (9) }$$
Table 2 showcases the performance of mild detection approaches for AD using two prominent pretrained networks, AlexNet and VGG19, in combination with MSVM and ANN classifiers. The findings indicate that AlexNet paired with MSVM achieves the highest accuracy among the tested methods. Notably, AlexNet with MSVM attains a remarkable 95.8% accuracy for the “Non-Demented” and “Very Mild Demented” categories, demonstrating its exceptional capability in distinguishing between mild and non-demented AD cases. This superior performance is attributed to AlexNet’s efficient feature extraction and the robust multiclass classification framework provided by MSVM.
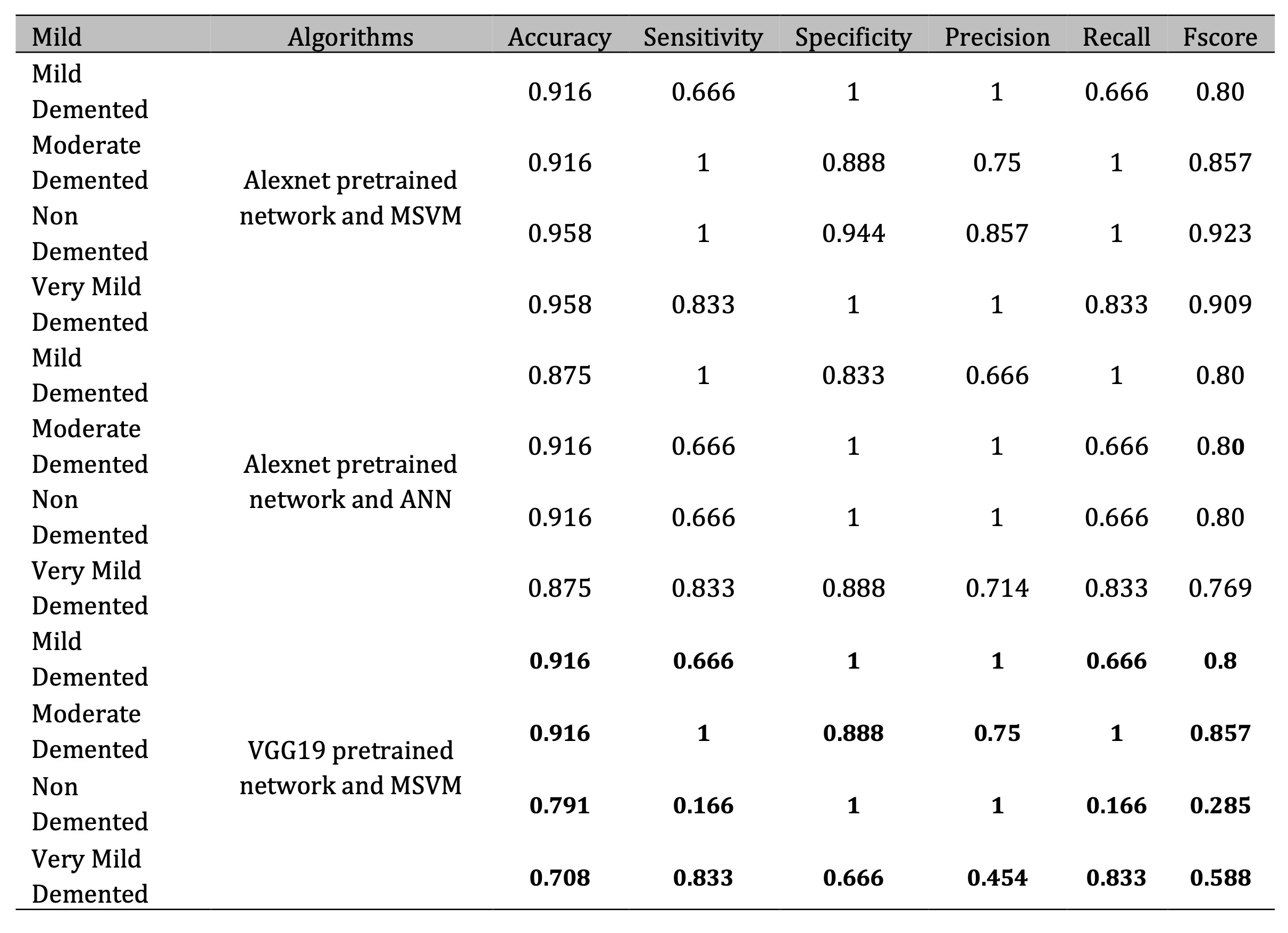
Table 2: Evaluation of proposed Alzheimer detection algorithms for AD datasets using pretrained network and MSVM/ANN
In contrast, VGG19 with MSVM delivers inconsistent results. While, it performs well for “Moderate Demented” cases (91.6% accuracy), its accuracy for “Non-Demented” and “Very Mild Demented” categories drops to 79.1% and 70.8%, respectively. This variability is likely due to VGG19’s deeper architecture, which may overfit smaller datasets and struggle with detecting subtle differences in early AD stages.
Discussion
The findings of this study reveal that the combination of the ResNet-18 pretrained network with ANN delivers the most effective results among the evaluated algorithms. This pairing consistently achieved the highest levels of accuracy, recall, and precision in identifying various AD stages. ResNet-18’s hierarchical structure, comprising 18 residual layers, addresses the vanishing gradient problem and facilitates the extraction of deep, abstract features, which are essential for medical imaging tasks. The ANN classifier enhances this architecture by efficiently managing the non-linear relationships within the extracted features. ResNet-18 has been widely recognized in prior research for its strong performance in medical imaging. For example, He et al. (2016) [36] demonstrated its superiority over deeper architectures, such as VGG-19, in both computational efficiency and accuracy. Similarly, Qin et al. (2022) [37] reported that ResNet-18, when paired with attention mechanisms, exhibited exceptional sensitivity in detecting early stages of neurodegenerative disorders. In this study, ResNet-18 with ANN outperformed AlexNet and VGG-19, particularly in terms of accuracy and robustness for moderate and very mild AD stages. These findings align with the observations of Bron et al. (2015) [10], who emphasized the adaptability of residual networks to complex medical datasets with diverse imaging features. Additionally, ANN’s role in optimizing classification performance is supported by Thai et al. (2012) [28], who highlighted its ability to handle high-dimensional feature spaces effectively. Overall, the synergy between ResNet-18 and ANN leverages the strengths of both frameworks, establishing it as a powerful solution for precise and early AD detection. Future research could explore its application to multi-modal imaging datasets and larger population cohorts to further validate its clinical utility.
Table 3 further evaluates AlexNet’s performance with ANN classifiers optimized using SGD and Adam. The Adam optimizer consistently outperforms SGD, particularly for the “Non-Demented” category, where it achieves an accuracy of 91.6% compared to SGD’s 70.8%. Adam’s dynamic learning rate adjustment plays a key role in optimizing training, especially for diverse datasets. Both optimizers excel in classifying “Moderate Demented” cases, achieving 100% accuracy, underscoring ANN’s effectiveness in detecting pronounced AD features in advanced stages.
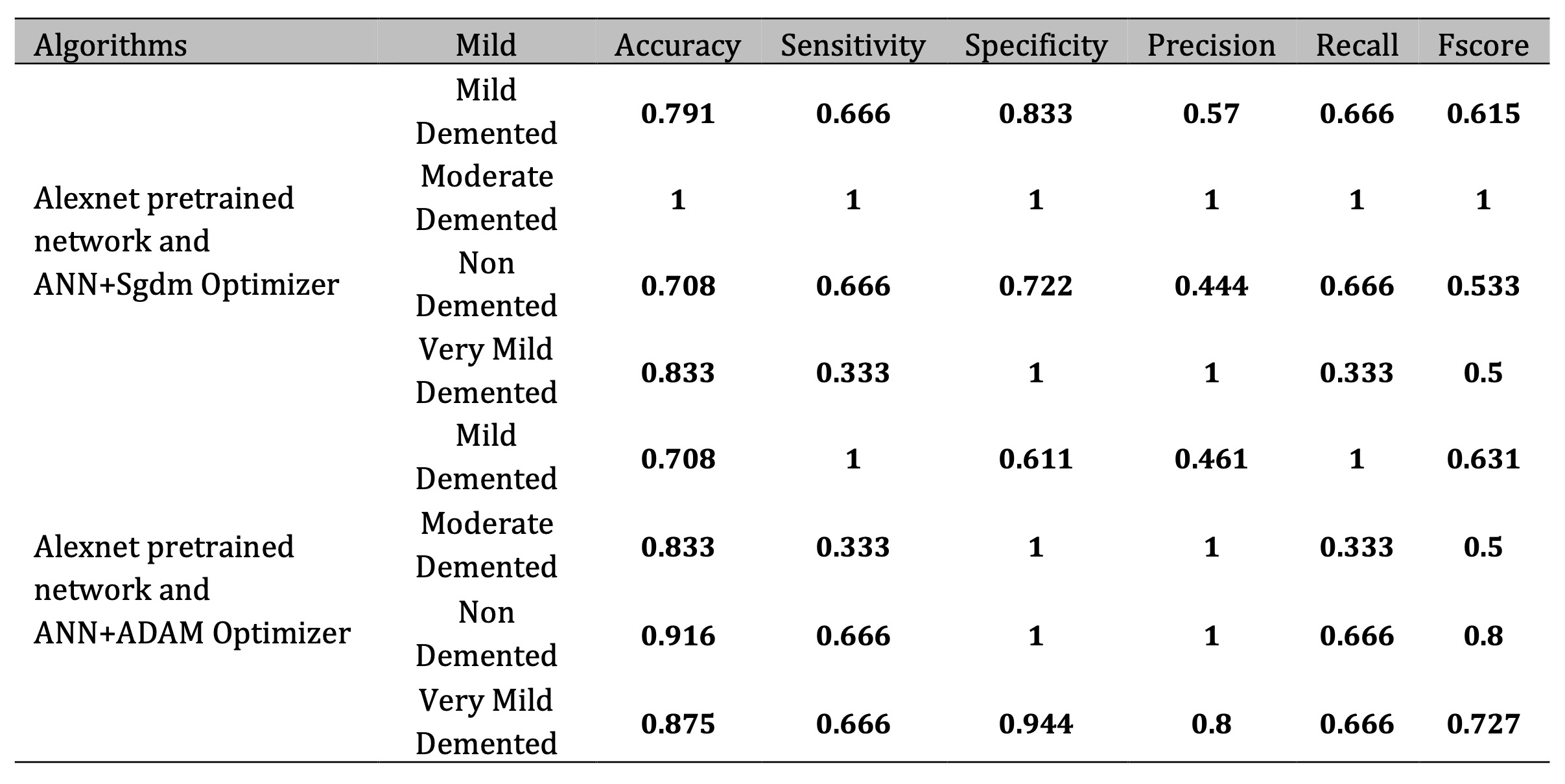
Table 3: Analysis based on Alexnet with ANNs optimized with SGD and Adam
The analysis highlights several key findings regarding the efficacy of various models and methods for detecting AD across different stages. The combination of AlexNet and MSVM emerges as the most effective approach, achieving the highest overall accuracy and precision, particularly in the detection of mild AD. On the other hand, VGG19 paired with MSVM shows notable proficiency in identifying moderate stages of AD. However, its deeper architecture appears to underperform in detecting early-stage AD, likely due to an increased risk of overfitting. Additionally, the integration of AlexNet with an ANN and the Adam optimizer significantly enhances classification accuracy, especially for mild and very mild categories of AD, demonstrating its potential for applications involving nuanced datasets. These findings align with previous research, such as He et al. (2016) [36], which highlighted the efficacy of shallow networks like AlexNet for feature-rich medical imaging tasks. Overall, the results provide compelling evidence that the AlexNet pretrained network, when paired with MSVM or ANN and the Adam optimizer, outperforms other advanced approaches for AD detection and classification.
Figures 7–11 provide valuable insights into the performance and accuracy of the proposed AD detection algorithms, which integrate various pretrained CNN architectures with different classifiers. Fig. 7 highlights the accuracy achieved by AlexNet when combined with ANN classifiers using SGD and Adam optimization techniques. The results indicate that the Adam optimizer consistently outperforms SGD across all datasets, particularly for detecting mild and very mild AD stages. These findings align with Kingma and Ba (2015) [38], who demonstrated that Adam’s dynamic learning rate adjustments significantly enhance optimization in deep networks. The high accuracy achieved validates the efficacy of combining AlexNet with ANN for detecting AD across multiple stages.
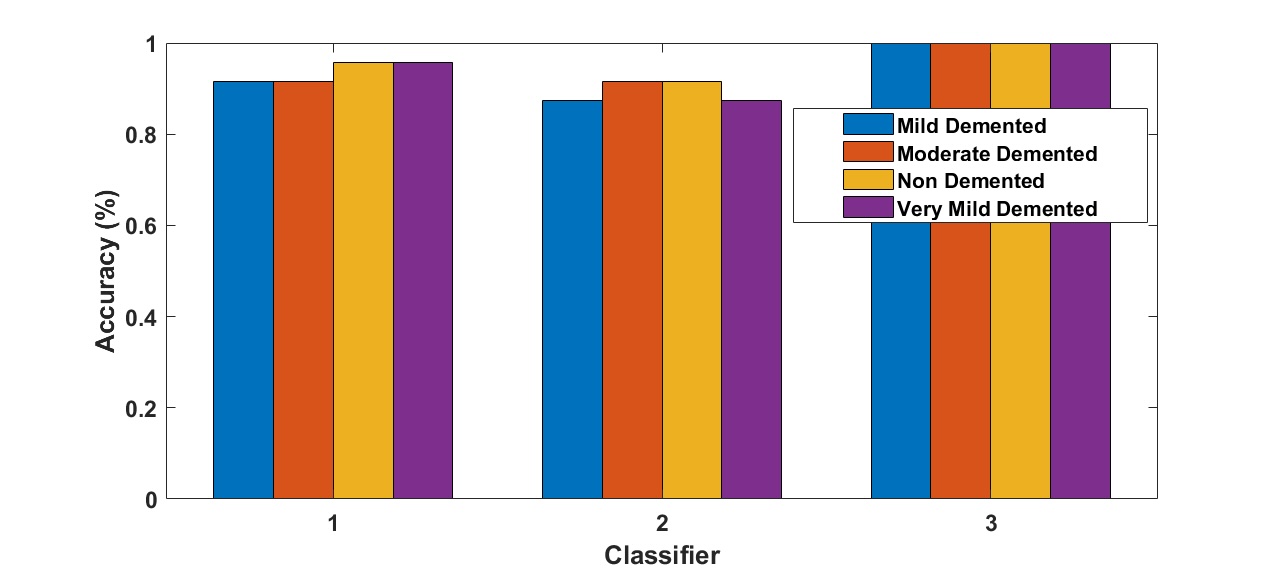
Fig. 7: The accuracy of Alzheimer detection algorithm using Alexnet pre-trained networks with ANNs for different optimizers.
Fig. 8 illustrates the confusion matrix for mild AD detection using AlexNet with MSVM. This matrix reveals strong classification performance, with high precision and recall values, showcasing the model’s reliability. The confusion matrix offers detailed insights by comparing true positive and negative predictions against their false counterparts, confirming MSVM’s robustness in managing multiclass classification tasks, as supported by Vapnik (1995) [39]. The accurate classification of mild AD stages further emphasizes the suitability of AlexNet for early-stage AD detection.
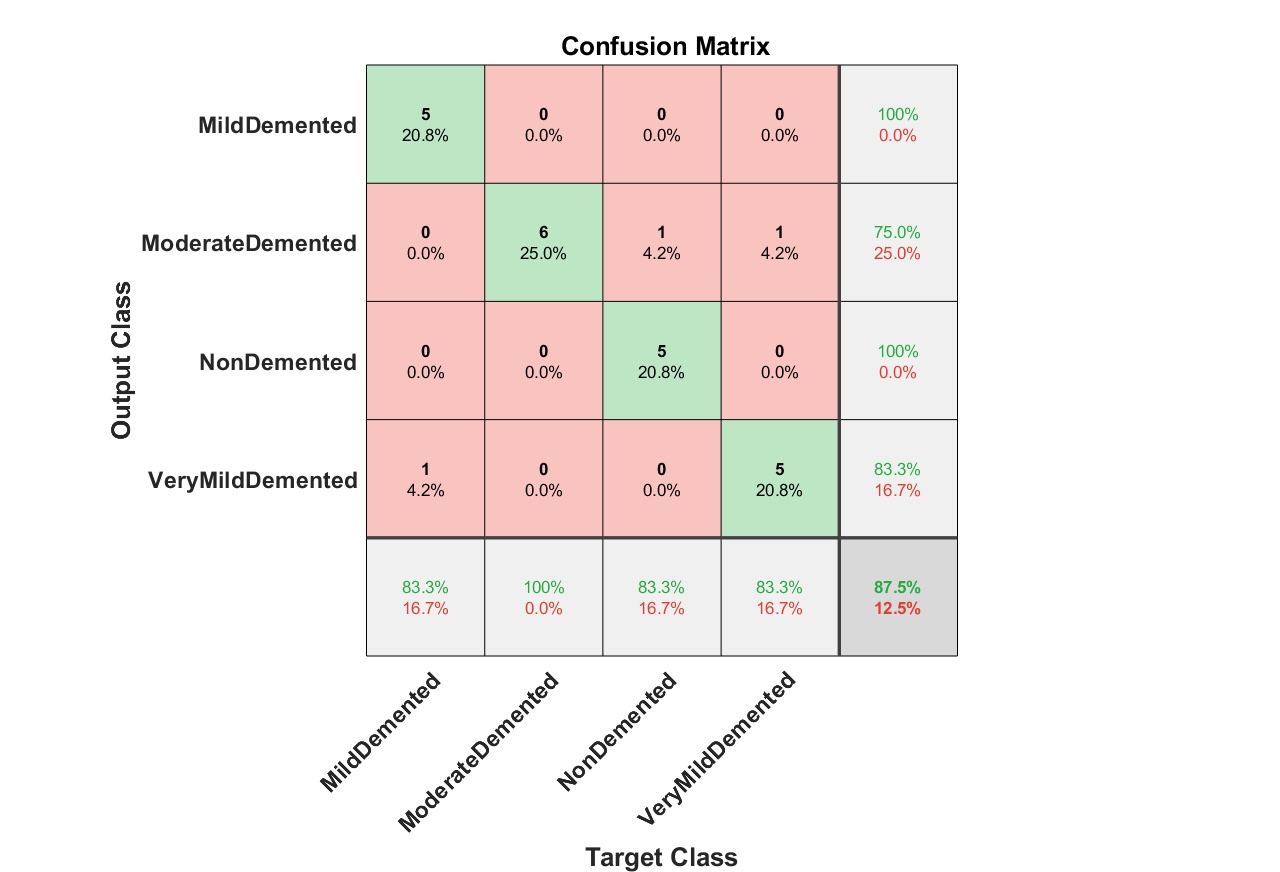
Fig. 8: Confusion matrix for mild detection of Alzheimer dataset images using AlexNet and MSVM.
Fig. 9 presents the confusion matrix for mild AD detection using AlexNet with ANN. Although slightly less robust than MSVM, ANN still achieves high levels of precision and sensitivity, underscoring its capability to handle non-linear and complex data patterns. These findings corroborate earlier research by Thai et al. (2012) [28], which highlighted ANN’s effectiveness in processing high-dimensional data.
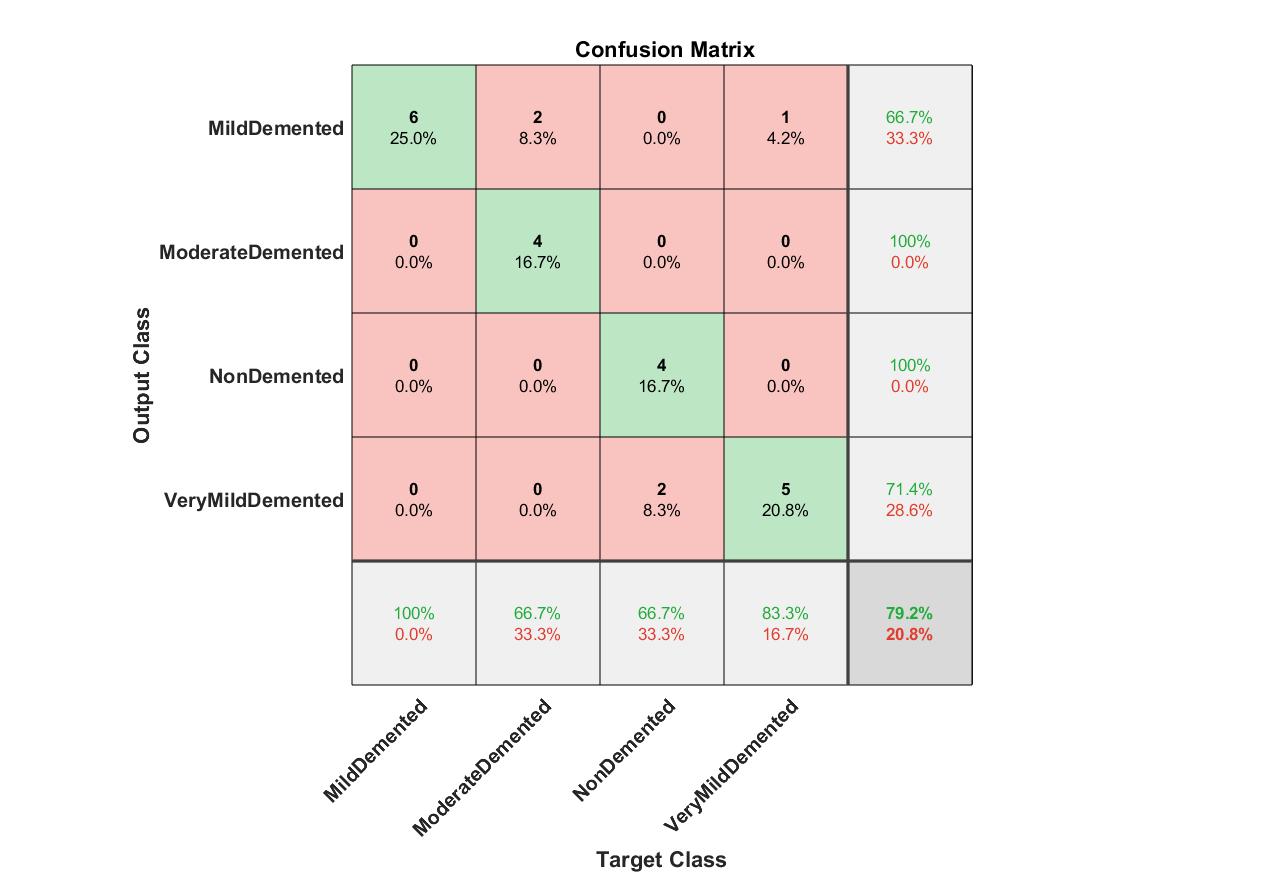
Fig. 9: Confusion matrix for mild detection of Alzheimer dataset images using AlexNet and ANN.
Fig. 10 (a–d) showcases detected samples from the AD datasets using AlexNet combined with MSVM for four categories: non-demented, very mild demented, mild demented, and moderate demented. These images demonstrate the model’s ability to distinguish between normal and AD-affected images. The visual outputs align with Bron et al. (2015) [10], who emphasized the importance of extracting detailed features for accurate classification in neurodegenerative disease datasets.
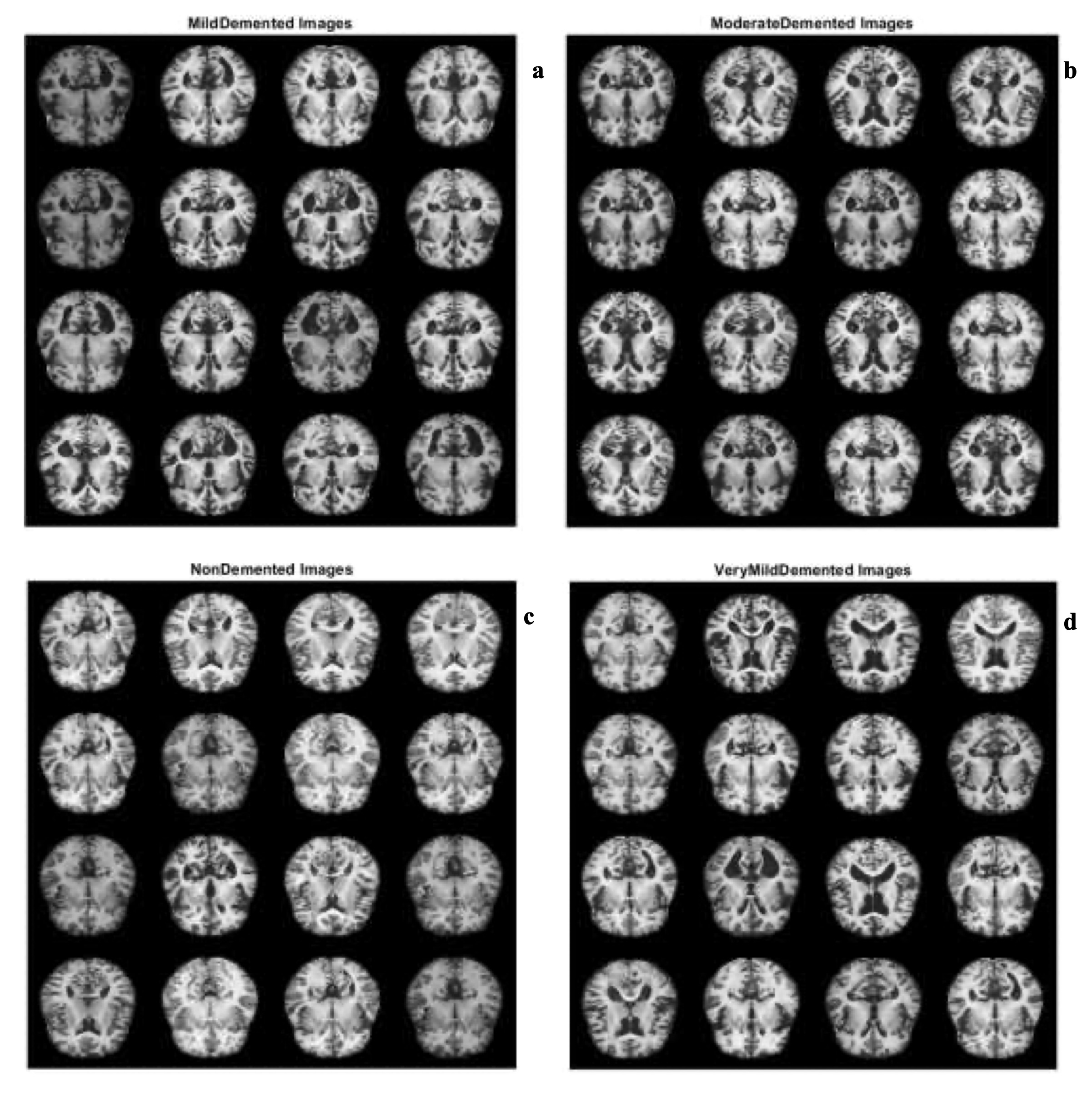
Fig. 10: Samples of a) Mild demented b) Moderate demented c) Non demented d) Very mild demented AD images using AleXnet pretrained CNN with MSVM.
Fig. 11 displays detected samples using AlexNet with ANN. The results confirm that ANN is highly effective in capturing subtle differences across AD categories, particularly for mild and moderate cases. These findings align with Amoroso et al. (2018) [40], who reported similar success in employing ANNs for hippocampal feature extraction in AD diagnosis.
Fig. 11: AD detection from dataset images using Alexnet pretrained network and ANN.
The findings presented in Figures 7–11 are consistent with prior studies in medical imaging and deep learning-based classification. The superior performance of the Adam optimizer (Fig. 7) reaffirms the conclusions of Kingma and Ba (2015) [38], who highlighted Adam’s efficiency in deep learning tasks. The confusion matrices in Figures 8 and 9 validate the reliability of AlexNet combined with MSVM and ANN, echoing the robustness noted by Vapnik (1995) [39] and Thai et al. (2012) [28]. Finally, the visual outputs in Figures 10 and 11 demonstrate the diagnostic precision of AlexNet pretrained models, aligning with the work of Bron et al. (2015) [10] and Amoroso et al. (2018) [40]. These results collectively highlight the strength of AlexNet when paired with MSVM and ANN classifiers in AD detection and classification. They reinforce the potential of such approaches to contribute significantly to advancements in automated neurodegenerative disease diagnosis.
Conclusion
The aim of the article is to early detect and diagnose the AD for timely intervention and improved patient outcomes. An approach based on DL is suggested for automated AD detection and classification. The features are extracted using CNN with three different networks including AlexNet, ResNet-18 and VGG-19. These features are analyzed using MSVM and ANN classifiers. Besides, the AD is detected and classified using SVM classifier with texture features. These approaches are tested using four different categories of demented AD images. These are non demented, mild demented, moderate demented and very mild demented. These approaches aimed to achieve robust classification performance for differentiation among normal and AD demented images. The proposed DL approach introduces the best results in terms of accuracy (91%), precision (95%), recall (90%), and F1-score. The results indicated that 7 texture features are selected for distinguishing between normal and mild AD disability. Future research is needed to explore the effectiveness of this approach on larger and more diverse datasets. Additionally, the integration with other neuroimaging modalities such as MRI scans could potentially enhance the diagnostic accuracy.
Abbreviations
DL (Deep Learning); AD (Alzheimer Disease); CAD (Computer Aided Detection); CNNs (Convolution Neural Networks); MSVM (Multi-Class Support Vector Machine); ANN (Artificial Neural Network); PDS (Power Spectral Density); HOS (Higher-Order Statistics); RBF (Radial Basis Function); SGD (Stochastic Gradient Descent); RP (True Positive); MRI (Magnetic Resonance Imaging); PET (Positron Emission Tomography); RN (True Negative); LN (False Negative); LP (False Positive).
Acknowledgements
The authors extend their appreciation to the King Salman center For Disability Research for funding this work through Research Group no KSRG-2023-513.
Funding
The authors extend their appreciation to the King Salman center For Disability Research for funding this work.
Author contributions
All Authors performed the numerical simulations, analyzed the data, wrote and revised the main manuscript text developed the final manuscript.
Availability of data and material
Requests for materials or code should be addressed to M. S. El_Tokhy.
Ethics Declarations
This article does not contain any studies involving animals or human participants performed by any authors.
Disclosure Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AI disclosure statement
No AI tools were used in the creation of this work
References
| 1 | Mohammed GA, Suhuai L, and Kamran S: Alzheimer's disease detection using deep learning on neuroimaging: a systematic review. Machine Learning Knowledge Extraction 2024;6(1):464-505.
https://doi.org/10.3390/make6010024 |
| 2 | Luo S, Li X, Li J: Automatic Alzheimer's disease recognition from MRI data using deep learning method. Journal of Applied Mathematics and Physics 2017;5(9).
|
| 3 | Waleed A: Alzheimer's disease diagnosis and classification using deep learning techniques. PeerJ Comput Sci. 2022;8(e1177)
https://doi.org/10.7717/peerj-cs.1177 |
| 4 | Prince M, Wimo A, Guerchet M, Ali GC, WuY T, Prina M: World Alzheimer Report 2015: The global impact of dementia. Alzheimer's Disease International 2015; 21m:1-84.
|
| 5 | Alzheimer's Association: Alzheimer's disease facts and figures. Alzheimer's & Dementia 2024;20(5):3708-3821.
https://doi.org/10.1002/alz.13809 |
| 6 | Klunk WE, Engler H, Nordberg A et al.: Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of Neurology 2004;55(3):306-319.
https://doi.org/10.1002/ana.20009 |
| 7 | Jack CR, Bennett DA, Blennow K et al.: NIA-AA research framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & Dementia 2018;14(4):535-562.
https://doi.org/10.1016/j.jalz.2018.02.018 |
| 8 | Suchitra S, Krishnasamy L, Poovaraghan RJ: A deep learning-based early alzheimer's disease detection using magnetic resonance images. Multimedia Tools and Applications 2024.
https://doi.org/10.1007/s11042-024-19677-9 |
| 9 | El-Assy AM, Amer HM, Ibrahim HM, Mohamed MA: A novel CNN architecture for accurate early detection and classification of Alzheimer's disease using MRI data. scientific reports 2024;24.
https://doi.org/10.1038/s41598-024-53733-6 |
| 10 | Bron EE, Smits M, van der Flier WM et al.: Standardized evaluation of algorithms for computer-aided diagnosis of dementia based on structural MRI: The CAD Dementia challenge. NeuroImage 2015;111:562-579.
https://doi.org/10.1016/j.neuroimage.2015.01.048 |
| 11 | Liu M, Zhang D, Shen D: Ensemble sparse classification of Alzheimer's disease. NeuroImage 2012;60(2):1106-1116.
https://doi.org/10.1016/j.neuroimage.2012.01.055 |
| 12 | El_Tokhy MS, Ismail I.M.: Classification of Welding Flaws in Gamma Radiography Images Based on Multi-scale Wavelet Packet Feature Extraction Using Support Vector Machine. Journal of Nondestructive Evaluation 2015;34(4).
https://doi.org/10.1007/s10921-015-0305-9 |
| 13 | Qin Z, Liu Z, Guo Q and Zhu P: 3D convolutional neural networks with hybrid attention mechanism for early diagnosis of Alzheimer's disease. Biomedical Signal Processing and Control 2022, 77.
https://doi.org/10.1016/j.bspc.2022.103828 |
| 14 | Nocetti D, Villalobos K, Marín N, Monardes M, Tapia B, Toledo MI, Villegas C: Radiation dose reduction and image quality evaluation for lateral lumbar spine projection. Heliyon 2023; 9(9).
https://doi.org/10.1016/j.heliyon.2023.e19509 |
| 15 | Kim N, Rowe BH, Raymond G, Jen H, Colman I, Jackson SA, Siminoski KG, Chahal AM, Folk D, Majumdar SR: Underreporting of vertebral fractures on routine chest radiography. American Journal of Roentgenology 2005;182(2):297-300.
https://doi.org/10.2214/ajr.182.2.1820297 |
| 16 | Suk HI, Lee SW, Shen D: Deep ensemble learning of sparse regression models for brain disease diagnosis. Medical Image Analysis 2017, 37:101-113.
https://doi.org/10.1016/j.media.2017.01.008 |
| 17 | El_Tokhy MS, Mahmoud II: Development of Digital Inspection Algorithms for X-Ray Radiography Casting Images. Russian Journal of Nondestructive Testing 2019, 55: 334-343.
https://doi.org/10.1134/S1061830919040053 |
| 18 | El_Tokhy MS, Saad MH: Automatic detection algorithm of defects in casting radiography images based on Cepstral coefficients. Arab Journal of Nuclear Sciences and Applications 2017;50(4):18-28.
|
| 19 | Amoroso N, La Rocca M, Bellotti R, Fanizzi A, Monaco A., Tangaro S: Alzheimer's disease diagnosis based on the Hippocampal Unified Multi-Atlas Network (HUMAN) algorithm. Neuroinformatics 2018;16(2):285-295.
|
| 20 | LeCun Y, Bengio Y, Hinton G: Deep learning. Nature 2015;521(7553):436-444.
https://doi.org/10.1038/nature14539 |
| 21 | Schmidhuber J: Deep learning in neural networks: An overview. Neural Networks 2015;61:85-117.
https://doi.org/10.1016/j.neunet.2014.09.003 |
| 22 | El_Tokhy MS: Development of optimum watermarking algorithm for radiography images. Computers & Electrical Engineering 2021;89.
https://doi.org/10.1016/j.compeleceng.2020.106932 |
| 23 | El_Tokhy MS: Robust Multimodal Biometric Authentication Algorithms Using Fingerprint, Iris and Voice Features Fusion. Journal of Intelligent & Fuzzy Systems 2021;40(1):647-672.
https://doi.org/10.3233/JIFS-200425 |
| 24 | Qiu S.: Multimodal deep learning for Alzheimer's disease dementia assessment. Nature Communications 2022;13.
|
| 25 | Qureshi SA, Hussain L, Ibrar U, Alabdulkreem E, Nour MK, Alqahtani MS, Nafie FM, Mohamed A, Mohammed GP, Duong TQ: Radiogenomic classification for MGMT promoter methylation status using multi-omics fused feature space for least invasive diagnosis through mpMRI scans. Scientific Reports 2023;13.
https://doi.org/10.1038/s41598-023-30309-4 |
| 26 | Zheng B, Yoon SW, Lam SS: Breast cancer diagnosis based on feature extraction using a hybrid of K-means and support vector machine algorithms. Expert Systems with Applications 2014;41(4):1476-1482.
https://doi.org/10.1016/j.eswa.2013.08.044 |
| 27 | Pange S, Lokhande S: Image retrieval system by using CWT and support vector machines. An International Journal (SIPIJ), Signal and Image Processing 2012, 3(3).
https://doi.org/10.5121/sipij.2012.3306 |
| 28 | Thai LH, Hai TS, Thuy NT: Image Classification using Support Vector Machine and Artificial Neural Network. I.J. Information Technology and Computer Science, IACSIT Press, Singapore 2012;5:32-38.
https://doi.org/10.5815/ijitcs.2012.05.05 |
| 29 | Korkmaza SA, Poyraz M: Least square support vector machine and minumum redundancy maximum relavance for diagnosis of breast cancer from breast microscopic images. Procedia-Social and Behavioral Sciences 2015, 174:4026-4031.
https://doi.org/10.1016/j.sbspro.2015.01.1150 |
| 30 | Lo C-S, Wang C-M: Support vector machine for breast MR image classification. Computers and Mathematics with Applications 2012;64(5):1153-1162.
https://doi.org/10.1016/j.camwa.2012.03.033 |
| 31 | Hassan MR, Fikry RM, Aly MI: Black box dynamic modeling of Co(II) ions removal from aqueous solution using modified maghemite nanoparticles by fixed‑bed column based on deep neural networks. Chemical Papers 2021;75:763-777.
https://doi.org/10.1007/s11696-020-01334-8 |
| 32 | Alzheimer's Dataset (4 class of Images) Online]. Available: https://www.kaggle.com/datasets/tourist55/alzheimers-dataset-4-class-of-images
|
| 33 | El_Tokhy MS: Development of precise forgery detection algorithms in digital radiography images using convolution neural network. Applied Soft Computing 2023, 138.
https://doi.org/10.1016/j.asoc.2023.110174 |
| 34 | El_Tokhy MS: An efficient digital pulse processing approach for identification of BGO and LSO scintillator crystals. Journal of Instrumentation 2023; 18.
https://doi.org/10.1088/1748-0221/18/03/P03036 |
| 35 | Yilan W, Xiaobing K, Yajun C: Robust and accurate detection of image copy-move forgery using PCET-SVD and histogram of block similarity measures. Journal of Information Security and Applications 2020; 54.
https://doi.org/10.1016/j.jisa.2020.102536 |
| 36 | He K, Zhang X, Ren S, Sun J: Deep residual learning for image recognition. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR) 2016;770-778.
https://doi.org/10.1109/CVPR.2016.90 |
| 37 | Qin Z, Liu Z, Guo Q, Zhu P: 3D convolutional neural networks with hybrid attention mechanism for early diagnosis of Alzheimer's disease. Biomedical Signal Processing and Control 2022;77.
https://doi.org/10.1016/j.bspc.2022.103828 |
| 38 | Kingma DP, Ba J: Adam: A method for stochastic optimization. Proceedings of the International Conference on Learning Representations (ICLR) 2015.
|
| 39 | Vapnik V: The nature of statistical learning theory. Springer 1995 DOI: 10.1007/978-1-4757-2440-0.
https://doi.org/10.1007/978-1-4757-2440-0 |
| 40 | Amoroso N, La Rocca M, Bellotti R et al.: Alzheimer's disease diagnosis based on the Hippocampal Unified Multi-Atlas Network (HUMAN) algorithm. Neuroinformatics 2018, 16(2),285-295.
|